Redox reactions
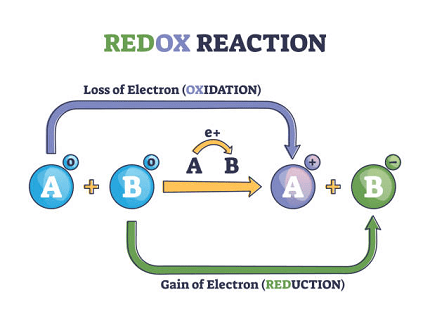
Short for oxidation-reduction reactions, are chemical reactions in which there is a transfer of electrons between species. In these reactions, one species undergoes oxidation (loses electrons) while another species undergoes reduction (gains electrons). This transfer of electrons is accompanied by changes in the oxidation numbers of the elements involved.
Key concepts related to redox reactions include oxidation numbers, half-reactions, and balancing the reaction equation.
- Oxidation and Reduction:
- Oxidation: The process in which a species loses electrons, resulting in an increase in its oxidation number.
- Reduction: The process in which a species gains electrons, resulting in a decrease in its oxidation number.
- Oxidation Numbers:
- Oxidation number (or oxidation state) is a concept that assigns a numerical value to each element in a compound or ion.
- It indicates the apparent charge an atom would have if the compound or ion was purely ionic.
- Rules and guidelines are used to determine oxidation numbers, such as the electronegativity of the elements and the nature of the chemical bonds.
- Half-Reactions:
- A redox reaction can be split into two half-reactions: the oxidation half-reaction and the reduction half-reaction.
- The oxidation half-reaction represents the species that undergoes oxidation, while the reduction half-reaction represents the species that undergoes reduction.
- Each half-reaction involves the transfer of electrons and may also involve other chemical species.
- Balancing Redox Reactions:
- Balancing redox reactions requires ensuring that the number of electrons lost in the oxidation half-reaction equals the number of electrons gained in the reduction half-reaction.
- Different methods can be used for balancing redox reactions, such as the oxidation number method and the half-reaction method.
- The balanced equation should satisfy the law of conservation of mass and charge.
- Applications of Redox Reactions:
- Redox reactions are fundamental in various chemical and biological processes, including combustion, corrosion, electrolysis, and energy production in cells.
- Redox reactions are essential in analytical chemistry, where they are used in titrations and determining the concentrations of species.
Understanding redox reactions is crucial in many areas of chemistry, such as inorganic chemistry, organic chemistry, electrochemistry, and biochemistry. It forms the basis for understanding and predicting the behavior of substances undergoing electron transfer, which is fundamental in numerous chemical reactions and natural processes.
The AIIMS (All India Institute of Medical Sciences) entrance examination is a highly competitive medical entrance exam in India. The chemistry syllabus for AIIMS typically covers a wide range of topics, including redox reactions. Redox reactions, also known as oxidation-reduction reactions, involve the transfer of electrons between species.
Here is a general outline of the redox reaction topics that may be included in the AIIMS chemistry syllabus:
- Introduction to Redox Reactions:
- Definition of redox reactions
- Oxidation and reduction processes
- Oxidation number concept
- Balancing Redox Reactions:
- Balancing redox reactions using the oxidation number method
- Half-reaction method for balancing redox reactions
- Redox Reactions in Aqueous Solutions:
- Electrolytes and nonelectrolytes
- Dissociation of ionic compounds in water
- Net ionic equations for redox reactions in solution
- Electrochemical Cells:
- Galvanic (voltaic) cells and electrolytic cells
- Standard reduction potential and the electrochemical series
- Cell potential, emf (electromotive force), and Gibbs free energy
- Electrolysis and Electroplating:
- Faraday’s laws of electrolysis
- Electrolysis of molten salts and aqueous solutions
- Electroplating and its applications
- Redox Titrations:
- Redox indicators
- Potentiometric titrations
- Applications of redox titrations in analytical chemistry
- Corrosion and Rusting:
- Types of corrosion
- Factors affecting corrosion
- Preventive measures for corrosion
It’s important to note that the AIIMS syllabus may vary slightly from year to year, so it’s recommended to refer to the official syllabus provided by the exam conducting authority for the most accurate and up-to-date information.
Additionally, it’s always beneficial to consult the specific study materials, textbooks, and reference books recommended for AIIMS preparation to ensure comprehensive coverage of the redox reaction topics in the syllabus.
What is Required AIIMS-SYLLABUS Chemistry syllabus Redox reaction
- Definition and Concepts:
- Redox reactions and their significance in chemical processes.
- Oxidation and reduction processes.
- Oxidation number concept and its application in determining the oxidation state of elements in compounds and ions.
- Balancing Redox Reactions:
- Balancing redox reactions using the oxidation number method.
- Balancing redox reactions using the half-reaction method.
- Understanding and applying the principles of conservation of mass and charge in balanced redox equations.
- Redox Reactions in Aqueous Solutions:
- Understanding the behavior of ionic compounds in aqueous solutions.
- Writing and balancing net ionic equations for redox reactions occurring in aqueous solutions.
- Electrochemical Cells:
- Understanding the principles of galvanic (voltaic) cells and electrolytic cells.
- Redox reactions occurring in electrochemical cells and their application in generating electricity or performing electrolysis.
- Concepts of standard reduction potential, electrochemical series, and cell potential.
- Electrolysis and Electroplating:
- Understanding Faraday’s laws of electrolysis.
- Electrolysis of molten salts and aqueous solutions.
- Applications of electrolysis, such as electroplating and extraction of metals.
- Corrosion and Rusting:
- Types of corrosion and factors influencing corrosion.
- Understanding the process of rusting and its prevention.
It is important to note that the AIIMS syllabus may undergo revisions, so it’s recommended to refer to the official syllabus or information provided by the exam conducting authority for the most accurate and up-to-date details. Additionally, referring to recommended textbooks and study materials specific to AIIMS preparation will help in covering the redox reaction topics thoroughly.
Case Study on AIIMS-SYLLABUS Chemistry syllabus Redox reaction
Introduction:
The AIIMS entrance examination is highly competitive and requires a thorough understanding of the chemistry syllabus, including the topic of redox reactions. In this case study, we will explore how a student, Priya, prepared for the AIIMS examination by focusing on the redox reaction syllabus.
Background:
Priya, a dedicated medical aspirant, aimed to excel in the AIIMS examination. She recognized the importance of the chemistry syllabus and the topic of redox reactions, as it plays a crucial role in various chemical and biological processes.
Study Approach:
To master the redox reaction syllabus, Priya followed a structured study approach that involved the following steps:
- Understanding Concepts: Priya began by developing a strong conceptual foundation. She studied the definitions and concepts related to redox reactions, including oxidation, reduction, and oxidation numbers. She grasped the significance of electron transfer and its connection to changes in oxidation states.
- Balancing Redox Reactions: Priya dedicated time to mastering the art of balancing redox reactions. She practiced using both the oxidation number method and the half-reaction method. She made sure to understand the principles of conservation of mass and charge while balancing the equations accurately.
- Applying Knowledge: Priya applied her knowledge of redox reactions to solve problems and practice questions. She solved a variety of problems, ranging from simple to complex, to reinforce her understanding of the topic. She also attempted previous years’ AIIMS question papers to familiarize herself with the exam pattern and types of questions asked.
- Exploring Applications: Priya delved into the practical applications of redox reactions. She studied electrochemical cells, including galvanic and electrolytic cells, and their relevance in generating electricity and performing electrolysis. She also learned about redox titrations and their applications in analytical chemistry.
- Corrosion and Rusting: Priya thoroughly studied corrosion and rusting, which are common examples of redox reactions in everyday life. She understood the different types of corrosion, factors influencing corrosion, and preventive measures. Additionally, she explored the process of rusting and its prevention techniques.
Results and Conclusion:
Priya’s focused approach and dedication to mastering the redox reaction syllabus proved beneficial in her preparation for the AIIMS examination. By thoroughly understanding the concepts, balancing reactions accurately, and exploring practical applications, she gained a strong command over the topic.
The case study of Priya emphasizes the significance of redox reactions in the AIIMS chemistry syllabus. A comprehensive understanding of this topic enables students to solve complex problems, apply knowledge to practical scenarios, and excel in the examination.
White paper on AIIMS-SYLLABUS Chemistry syllabus Redox reaction
Title:
Understanding Redox Reactions: A Comprehensive Analysis of the AIIMS Chemistry Syllabus
Abstract:
This white paper provides a comprehensive analysis of the redox reaction topic in the AIIMS (All India Institute of Medical Sciences) chemistry syllabus. Redox reactions, also known as oxidation-reduction reactions, play a vital role in various chemical and biological processes. This paper aims to elucidate the key concepts, practical applications, and significance of redox reactions in the context of the AIIMS entrance examination.
- Introduction:
- Overview of the AIIMS examination and its chemistry syllabus.
- Importance of redox reactions in chemical and biological systems.
- Redox Reactions: Conceptual Understanding:
- Definition and fundamental principles of redox reactions.
- Oxidation and reduction processes.
- Oxidation numbers and their application in determining oxidation states.
- Balancing Redox Reactions:
- Methods for balancing redox reactions, including the oxidation number method and the half-reaction method.
- Detailed step-by-step procedures for balancing equations using each method.
- Conservation of mass and charge during the balancing process.
- Redox Reactions in Aqueous Solutions:
- Behavior of ionic compounds in aqueous solutions.
- Writing and balancing net ionic equations for redox reactions in solution.
- Understanding spectator ions and their role in net ionic equations.
- Electrochemical Cells:
- Introduction to galvanic (voltaic) cells and electrolytic cells.
- Cell potential, electromotive force (emf), and the Nernst equation.
- Standard reduction potentials and the electrochemical series.
- Applications of electrochemical cells in generating electricity and performing electrolysis.
- Redox Titrations:
- Redox indicators and their role in titrations.
- Potentiometric titrations and their significance in analytical chemistry.
- Applications of redox titrations in determining the concentration of species.
- Corrosion and Rusting:
- Types of corrosion and factors influencing corrosion.
- Understanding the process of rusting and its prevention techniques.
- Significance of corrosion prevention in various industries.
- Conclusion:
- Recapitulation of the key concepts covered in the AIIMS chemistry syllabus regarding redox reactions.
- Emphasizing the practical applications and relevance of redox reactions in different fields.
- Importance of a strong understanding of redox reactions for success in the AIIMS entrance examination.
This white paper provides a comprehensive overview of the redox reaction topic within the AIIMS chemistry syllabus. It aims to equip students with a solid conceptual foundation, problem-solving skills, and practical applications of redox reactions in preparation for the AIIMS examination. By understanding the principles and applications of redox reactions, aspiring medical students can confidently tackle related questions and excel in their pursuit of medical education.
Note:
This white paper is for informational purposes only and does not replace official AIIMS syllabus documentation. Students are advised to refer to the official AIIMS syllabus and consult recommended study materials for accurate and up-to-date information.
