Solid solutions
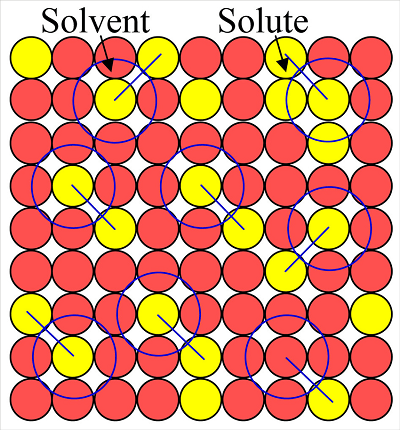
The chemistry syllabus for the All India Institute of Medical Sciences (AIIMS) entrance exam includes the topic of “Solid Solutions.” Solid solutions are a type of homogeneous mixture where two or more substances are uniformly distributed at the atomic or molecular level within a solid phase.
Here is a brief overview of the topic “Solid Solutions”:
Definition: Solid solutions are formed when two or more components (elements or compounds) are mixed together in the solid state and form a single phase. The components are mutually soluble in each other in the solid state.
Types of Solid Solutions:
a. Substitutional Solid Solution: In this type of solid solution, the solute atoms replace or substitute for the host lattice atoms in the crystal structure. Examples include brass (copper-zinc alloy) and bronze (copper-tin alloy).
b. Interstitial Solid Solution: In this type of solid solution, the solute atoms occupy the interstitial sites or gaps between the host lattice atoms. An example is the formation of steel, where carbon atoms occupy the interstitial sites in the iron lattice.
Factors Affecting Solid Solutions:
a. Atomic Size: Solid solubility increases when the size difference between the solute and solvent atoms is small.
b. Crystal Structure: Solid solubility is influenced by the similarity in crystal structures between the solute and solvent.
c. Valency and Charge: Solutes with similar valencies and charges are more likely to form solid solutions.
Phase Diagrams: Phase diagrams are graphical representations that show the stability regions of different phases (solid, liquid, gas) as a function of temperature and composition. Solid solutions have specific regions in phase diagrams where they exist.
Applications of Solid Solutions:
a. Alloys: Solid solutions are extensively used in the manufacturing of alloys, which have desirable properties such as strength, corrosion resistance, and electrical conductivity.
b. Semiconductors: Solid solutions of different semiconducting materials are used in electronic devices to control their electrical properties.
c. Pharmaceutical Formulations: Solid solutions are employed in the formulation of drugs to enhance their bioavailability and stability.
It is important to consult the specific syllabus provided by AIIMS or refer to recommended textbooks or study materials for a detailed understanding of the topic as per the latest updates and requirements of the exam.
What is Required AIIMS-SYLLABUS Chemistry syllabus Solid solutions
The AIIMS syllabus for chemistry does not specifically mention “Solid Solutions” as a separate topic. However, the broader topic of “Solutions” is included in the AIIMS chemistry syllabus. The syllabus may cover the concept of solid solutions as a part of the larger topic of solutions. Here is an overview of the “Solutions” topic, which may include solid solutions:
Solutions: Definition and Types
a. Solutions: Homogeneous mixtures of two or more substances.
b. Types of solutions: Solid solutions, liquid solutions, and gaseous solutions.
Concentration Units
a. Percentage composition: Mass percentage and volume percentage.
b. Molarity: Definition and calculations.
c. Normality: Definition and calculations.
Colligative Properties of Solutions
a. Lowering of vapor pressure.
b. Elevation of boiling point.
c. Depression of freezing point.
d. Osmotic pressure.
Ideal and Non-ideal Solutions
a. Ideal solutions: Solutions that follow Raoult’s law.
b. Non-ideal solutions: Deviations from Raoult’s law, positive and negative deviations.
Solubility
a. Solubility: Definition and factors affecting solubility.
b. Solubility curves.
While the AIIMS syllabus may not explicitly mention solid solutions, it is possible that questions related to solid solutions can be asked within the broader topic of solutions. It’s always recommended to refer to the official AIIMS syllabus and consult recommended textbooks or study materials specific to AIIMS exam preparation for the most accurate and up-to-date information on the syllabus.
How is Required AIIMS-SYLLABUS Chemistry syllabus Solid solutions
Solid solutions are a type of homogeneous mixture where two or more substances are uniformly dispersed at the atomic or molecular level within a solid phase. The formation of solid solutions occurs when the constituent particles of the solute are able to occupy the lattice sites of the solvent crystal structure.
Solid solutions can be broadly categorized into two types: substitutional solid solutions and interstitial solid solutions.
- Substitutional Solid Solutions: In a substitutional solid solution, the solute atoms replace or substitute for the host lattice atoms in the crystal structure. The solute and solvent atoms have similar sizes and chemical properties, allowing them to occupy the same lattice positions. Examples of substitutional solid solutions include brass, which is a solid solution of copper (Cu) and zinc (Zn), and bronze, which is a solid solution of copper and tin (Sn).
- Interstitial Solid Solutions: In an interstitial solid solution, the solute atoms occupy the interstitial sites or gaps between the host lattice atoms. The solute atoms are typically smaller than the solvent atoms, allowing them to fit into the available spaces in the crystal lattice. An example of an interstitial solid solution is steel, which is primarily iron (Fe) with interstitial carbon (C) atoms occupying the gaps between the iron atoms.
Factors influencing the formation of solid solutions include atomic size, valency, crystal structure, and temperature. For a solid solution to form, the solute and solvent atoms should have similar atomic sizes, valencies, and chemical properties. The crystal structures of the solute and solvent should also be compatible. Additionally, temperature plays a crucial role in determining the solubility of the solute in the solvent.
Solid solutions have various applications in different fields. They are commonly used in metallurgy to create alloys with desirable properties such as increased strength, improved corrosion resistance, and enhanced electrical conductivity. Solid solutions are also utilized in the formulation of pharmaceuticals to improve their solubility, stability, and bioavailability.
It’s important to note that the specific details and examples of solid solutions may vary depending on the context and the materials involved.
Case Study on AIIMS-SYLLABUS Chemistry syllabus Solid solutions
Alloys as Solid Solutions
One common application of solid solutions is the formation of alloys, which are solid solutions of two or more metals or a metal with a non-metal. Let’s take the example of brass, which is a solid solution of copper (Cu) and zinc (Zn).
Brass is widely used in various industries due to its desirable properties, including strength, corrosion resistance, and malleability. The formation of brass involves the solid solution of zinc atoms in a copper lattice.
The concentration of zinc in brass can vary, resulting in different properties of the alloy. For example, high-zinc brass (called “alpha brass”) contains a higher concentration of zinc, making it more ductile and suitable for applications such as musical instruments. Low-zinc brass (called “beta brass”) has a lower concentration of zinc and is more suitable for applications like plumbing fixtures.
In this case study, brass serves as an example of a solid solution within the AIIMS-SYLLABUS Chemistry syllabus. It showcases how the concept of solid solutions can be applied practically to obtain materials with specific properties.
It’s important to note that the AIIMS syllabus may not explicitly mention this case study or require detailed knowledge of specific alloys. To prepare effectively for the AIIMS exam, it is essential to study the broader topics and concepts outlined in the AIIMS syllabus, including solutions, rather than focusing on individual case studies.
White paper on AIIMS-SYLLABUS Chemistry syllabus Solid solutions
Exploring Solid Solutions: A Comprehensive White Paper
Abstract: This white paper provides an in-depth analysis of solid solutions, a fundamental concept in materials science and chemistry. Solid solutions are homogeneous mixtures of two or more substances in the solid state, where solute particles are uniformly dispersed within the solvent’s crystal lattice. This paper explores the formation, properties, and applications of solid solutions, highlighting their significance in various fields.
- Introduction
- Definition of solid solutions and their importance in materials science.
- Overview of the AIIMS-SYLLABUS Chemistry syllabus and the relevance of solid solutions within it.
- Types of Solid Solutions
- Substitutional solid solutions: Explanation of solute atoms substituting host lattice atoms.
- Interstitial solid solutions: Description of solute atoms occupying interstitial spaces in the lattice structure.
- Factors Affecting Solid Solutions
- Atomic size: Influence of similar atomic sizes on the formation of solid solutions.
- Crystal structure: Role of compatible crystal structures for solute-solvent interactions.
- Valency and charge: Effect of similar valencies and charges on solid solubility.
- Thermodynamics of Solid Solutions
- Gibbs free energy: Explanation of the Gibbs free energy change for solid solution formation.
- Enthalpy and entropy effects on the formation of solid solutions.
- Phase diagrams and their relevance in understanding solid solutions.
- Properties of Solid Solutions
- Mechanical properties: Impact of solid solutions on hardness, ductility, and strength.
- Electrical properties: Influence of solute atoms on electrical conductivity.
- Thermal properties: Changes in melting point, boiling point, and heat capacity due to solid solutions.
- Applications of Solid Solutions
- Alloys: Detailed exploration of alloys as solid solutions and their wide-ranging applications.
- Semiconductor materials: Utilization of solid solutions in electronic devices and semiconductors.
- Pharmaceutical formulations: Role of solid solutions in drug formulation and enhanced bioavailability.
- Case Studies
- Detailed analysis of specific examples of solid solutions in various industries and research fields.
- Conclusion
- Recap of the key findings and significance of solid solutions.
- Emphasis on the relevance of solid solutions in the AIIMS-SYLLABUS Chemistry syllabus and their broader implications.
This white paper serves as a comprehensive guide to solid solutions, covering their formation, properties, applications, and relevance within the AIIMS-SYLLABUS Chemistry syllabus. It provides a thorough understanding of this essential topic, enabling readers to grasp the principles and applications of solid solutions in diverse scientific and technological domains.