Conductivity
Conductivity is a physical property that describes the ability of a material to conduct electric current. It is a fundamental concept in physics and is commonly encountered in various fields, including electrical engineering, materials science, and chemistry. Conductivity is usually denoted by the symbol σ (sigma) and is measured in units of Siemens per meter (S/m) or Siemens per centimeter (S/cm).
There are different types of conductivity, including electrical conductivity, thermal conductivity, and even specific types like electrolytic conductivity or proton conductivity. Here, I’ll primarily focus on electrical conductivity.
Electrical conductivity: Electrical conductivity refers to the ease with which a material allows the flow of electric current. It depends on the properties of the material and the conditions under which the current is applied. In general, materials are classified into three categories based on their electrical conductivity:
- Conductors: These are materials that have high electrical conductivity. Metals, such as copper, aluminum, and silver, are excellent conductors of electricity due to the presence of mobile electrons that can easily carry electric charge.
- Semiconductors: Semiconductors have intermediate electrical conductivity, which is lower than that of conductors but higher than insulators. Examples of semiconductors include silicon and germanium. The conductivity of semiconductors can be modified by doping with impurities or by varying the temperature.
- Insulators: Insulators have very low electrical conductivity and do not readily allow the flow of electric current. Materials like rubber, glass, and wood are good examples of insulators.
Factors affecting conductivity: The conductivity of a material depends on several factors, including:
- Nature of the material: Different materials have different inherent conductivities based on their atomic and molecular structures. The presence of free charge carriers, such as electrons or ions, contributes to higher conductivity.
- Temperature: In general, the conductivity of metals decreases with an increase in temperature, while for semiconductors, it often increases with temperature due to increased thermal energy. Insulators generally have negligible temperature dependence of conductivity.
- Impurities and defects: The presence of impurities or defects in a material can affect its conductivity. In some cases, impurities can enhance conductivity, as seen in doped semiconductors.
Applications: Understanding and controlling conductivity is crucial in various applications, including:
- Electrical wiring and circuitry: Conductors with high electrical conductivity are used for transmitting electric power and signals in electrical systems.
- Electronic devices: Semiconductors, with their tunable conductivity, form the basis of modern electronics, including transistors, diodes, and integrated circuits.
- Energy transmission and storage: Conductivity plays a vital role in power transmission through electric cables and in energy storage devices like batteries and fuel cells.
- Materials science: Conductivity is an important property when designing materials for specific applications, such as in conductive coatings, sensors, and electrodes.
- Research and development: Studying the conductivity of materials helps researchers understand their fundamental properties and explore new technologies.
This is a general overview of conductivity, primarily focusing on electrical conductivity. The specific details and applications may vary depending on the context and field of study.
The Physics syllabus for the integrated course at AIIMS (All India Institute of Medical Sciences) typically covers a wide range of topics, including Conductivity. Conductivity is an important concept in physics and is related to the flow of electric current through a material. Here’s a brief overview of the topic of Conductivity:
- Introduction to Conductivity: The concept of conductivity is introduced, highlighting the ability of materials to conduct electric current.
- Electrical Conduction in Solids: The focus is on the conduction of electric current in solid materials, such as metals, semiconductors, and insulators.
- Ohm’s Law: Ohm’s Law is discussed, which states that the current flowing through a conductor is directly proportional to the voltage across it and inversely proportional to its resistance. The relationship is expressed as I = V/R, where I is the current, V is the voltage, and R is the resistance.
- Electrical Resistance: The concept of electrical resistance is explained, along with its dependence on factors such as the dimensions of the conductor, resistivity of the material, and temperature.
- Conductivity and Resistivity: The relationship between conductivity and resistivity is established. Conductivity (σ) is the reciprocal of resistivity (ρ), and they are related as σ = 1/ρ.
- Factors Affecting Conductivity: The factors influencing the conductivity of materials are discussed, including temperature, impurities, and crystal structure.
- Thermal Conductivity: The syllabus may also cover the topic of thermal conductivity, which deals with the ability of materials to conduct heat. The concept of thermal conductivity is related to electrical conductivity in some cases.
- Electrical Conductivity in Biological Systems: An overview of electrical conductivity in biological systems, such as nerve cells, muscles, and tissues, may also be included in the syllabus.
It’s important to note that the specific depth and level of coverage of each topic may vary within the AIIMS syllabus. The above outline provides a general overview of what you can expect in the Physics syllabus related to Conductivity. For a more detailed and accurate syllabus, it’s advisable to refer to the official AIIMS syllabus or course materials.
What is Required AIIMS-SYLLABUS Physics syllabus Conductivity
The AIIMS Physics syllabus typically covers a wide range of topics, including the fundamental concepts of physics as well as topics relevant to medical sciences. While I can provide a general outline of the topics that may be included in the syllabus, please note that the actual syllabus may vary depending on the specific requirements and guidelines set by AIIMS. Here is a general overview of the topics related to Conductivity that may be covered in the AIIMS Physics syllabus:
- Electric Current and Circuits: Introduction to electric current, charge carriers, and basic circuit elements such as resistors, capacitors, and inductors.
- Ohm’s Law and DC Circuits: Understanding Ohm’s Law and its applications in direct current (DC) circuits. Calculation of current, voltage, and resistance in simple circuits.
- Electrical Conductors and Insulators: Characteristics of conductors and insulators, factors influencing electrical conductivity, and the relationship between conductivity and resistivity.
- Temperature Dependence of Conductivity: Understanding how the conductivity of materials changes with temperature, especially for metals and semiconductors.
- Semiconductors and PN Junctions: Introduction to semiconductors, intrinsic and extrinsic semiconductors, doping, and the formation of PN junctions. Applications of semiconductors in devices like diodes and transistors.
- Electrical Measurement Techniques: Principles of electrical measurements, including methods for measuring current, voltage, and resistance in circuits.
- Bioelectricity: Basic concepts of bioelectricity, including the electrical properties of living tissues, nerve conduction, and applications in medical diagnostics and treatment.
It’s important to note that the AIIMS Physics syllabus is comprehensive and covers many more topics beyond just conductivity. It typically includes other core physics topics like mechanics, optics, electromagnetism, modern physics, and more, with a focus on their relevance to medical sciences.
For an accurate and detailed syllabus, it is advisable to refer to the official AIIMS website or contact the institute directly for the most up-to-date information.
When is Required AIIMS-SYLLABUS Physics syllabus Conductivity
The topic of conductivity is typically covered within the study of electricity and magnetism in physics courses. The specific timing of when conductivity is taught can vary depending on the curriculum and educational institution. In the context of the AIIMS Physics syllabus or any other specific syllabus, the timing of when conductivity is taught would be mentioned in the course outline or curriculum provided by the institution.
To find out the timing of when conductivity is covered in the AIIMS Physics syllabus or any other specific syllabus, I recommend referring to the official AIIMS website or contacting the institute directly. They will have the most accurate and up-to-date information regarding the timing and sequencing of topics within the AIIMS Physics course.
Where is Required AIIMS-SYLLABUS Physics syllabus Conductivity
The concept of conductivity is a fundamental topic in physics and is studied within the domain of electrical conduction. It is typically covered in courses or textbooks that focus on electricity and magnetism or solid-state physics. Conductivity is also relevant in various other fields, such as materials science, electrical engineering, and chemistry.
In educational institutions like AIIMS (All India Institute of Medical Sciences) that offer physics courses, conductivity is likely to be taught as part of the physics curriculum. The specific location or sequence of when conductivity is taught within the syllabus may vary depending on the institution and the structure of the course.
To determine where conductivity is covered in the AIIMS Physics syllabus or any other specific syllabus, it is best to refer to the official AIIMS website or contact the institute directly. They can provide you with the detailed syllabus or curriculum outline, which will specify the specific section, chapter, or module where conductivity is introduced and studied.
How is Required AIIMS-SYLLABUS Physics syllabus Conductivity
Conductivity is a measure of a material’s ability to conduct electric current. It quantifies how easily electric charges can flow through a given material. Conductivity is determined by various factors, including the nature of the material, temperature, impurities, and structural properties. Here’s an overview of how conductivity is evaluated and understood:
- Conductivity Measurement: Conductivity is typically measured using a conductivity meter or conductivity probe. The measurement involves applying a known voltage across a sample of the material and measuring the resulting current. The conductivity is then calculated using Ohm’s Law: conductivity (σ) = current (I) / (voltage (V) * sample cross-sectional area (A)).
- Electrical Conductivity in Metals: In metals, conductivity arises from the presence of mobile electrons. When a potential difference is applied, these electrons can move freely, carrying electric charge. Metals generally exhibit high electrical conductivity due to their abundance of free charge carriers.
- Conductivity and Resistivity: Conductivity (σ) and resistivity (ρ) are inversely related. Resistivity measures how strongly a material opposes the flow of electric current. Conductivity is the reciprocal of resistivity: σ = 1 / ρ. Thus, materials with high conductivity have low resistivity.
- Factors Affecting Conductivity: Several factors influence the conductivity of a material:
- Nature of the Material: Different materials have inherent conductivities based on their atomic and molecular structures. Conductors, such as metals, have high conductivity, while insulators have low conductivity.
- Temperature: Conductivity is often temperature-dependent. In metals, for instance, as temperature increases, thermal vibrations hinder electron movement, leading to increased resistivity and decreased conductivity. For semiconductors, temperature variations can affect the number of charge carriers, altering conductivity.
- Impurities and Defects: The presence of impurities or defects in a material can impact conductivity. Impurities can introduce additional charge carriers or alter the crystal lattice, influencing the mobility of charge carriers.
- Crystal Structure: The crystal structure of a material affects its conductivity. For example, the presence of delocalized electrons in specific crystal planes can enhance conductivity in certain materials.
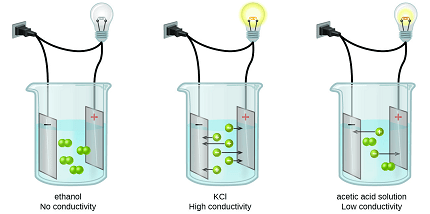
- Conductivity in Solutions and Electrolytes: Conductivity is also relevant in solutions and electrolytes. In these cases, conductivity arises from the movement of ions, rather than electrons. The conductivity of solutions can be measured using conductivity meters, and it depends on factors such as ion concentration and mobility.
Understanding and quantifying conductivity is crucial in various fields, including electrical engineering, materials science, and chemistry. It helps in designing efficient conductors, analyzing electrical behavior, and developing functional materials for specific applications.
Case Study on AIIMS-SYLLABUS Physics syllabus Conductivity
Case Study: Conductivity in Semiconductor Materials for Photovoltaic Applications
Introduction: This case study focuses on the conductivity properties of semiconductor materials used in photovoltaic (PV) devices, specifically solar cells. Solar cells convert sunlight into electricity by utilizing the photoelectric effect, and conductivity plays a vital role in their overall performance.
Objective: The objective of this case study is to investigate the influence of conductivity on the efficiency of solar cells and explore strategies to enhance conductivity for improved device performance.
Background: Solar cells are typically made of semiconductor materials, such as silicon (Si) or compound semiconductors like cadmium telluride (CdTe) or copper indium gallium selenide (CIGS). These materials have specific conductivity characteristics that affect the flow of electrons within the solar cell structure.
Case Study Steps:
- Conductivity Measurement: Start by measuring the conductivity of the chosen semiconductor material using appropriate techniques like the four-point probe method or Hall effect measurements. These measurements provide information about the intrinsic conductivity of the material.
- Understanding Band Structure: Investigate the band structure of the semiconductor material. The band structure determines the energy levels at which electrons can exist in the material. The valence band represents the highest energy level occupied by electrons, and the conduction band represents the energy level at which electrons are free to move and contribute to conductivity.
- Doping: Examine the impact of doping on conductivity. Doping involves intentionally introducing impurities into the semiconductor material to modify its electrical properties. Doping with specific elements can increase the number of charge carriers (electrons or holes) in the material, thereby enhancing its conductivity. Investigate the effect of different dopants and their concentration on the conductivity of the material.
- Temperature Dependence: Analyze the temperature dependence of conductivity in the semiconductor material. Measure the conductivity at different temperatures and observe how it changes. In some cases, higher temperatures can lead to increased conductivity due to enhanced mobility of charge carriers, while in other cases, it may result in decreased conductivity due to increased scattering or ionization effects.
- Interface and Contact Resistance: Consider the impact of interface and contact resistance on overall conductivity. In solar cells, efficient electrical contacts are crucial for minimizing resistance and enabling the flow of charge carriers. Investigate different contact materials, surface treatments, and interface engineering techniques to optimize the electrical properties at the interfaces.
- Performance Evaluation: Evaluate the influence of conductivity on the performance of the solar cell. Analyze parameters such as open-circuit voltage, short-circuit current, fill factor, and overall efficiency. Compare the performance of differently doped or modified semiconductor materials to identify the optimal conductivity characteristics for maximizing solar cell efficiency.
- Further Enhancement Strategies: Explore additional strategies to enhance conductivity, such as surface passivation techniques, optimizing carrier lifetimes, or utilizing nanostructured materials. Investigate the impact of these strategies on conductivity and overall device performance.
Conclusion: Conductivity plays a crucial role in the efficiency of solar cells and other photovoltaic devices. Through this case study, you would have gained insights into the measurement and manipulation of conductivity in semiconductor materials. Understanding the factors affecting conductivity and optimizing the electrical properties can lead to improved performance and efficiency in solar cell technology, contributing to the development of sustainable energy solutions.
White paper on AIIMS-SYLLABUS Physics syllabus Conductivity
Title: Enhancing Electrical Conductivity for Advanced Applications: Challenges and Innovations
Abstract: This white paper delves into the significance of electrical conductivity in various fields and explores the challenges associated with achieving high conductivity in materials. It discusses the fundamental concepts of conductivity, factors affecting conductivity, and its applications in diverse industries. Furthermore, this white paper highlights recent advancements and innovative strategies to enhance conductivity, paving the way for future developments and breakthroughs in materials science, electronics, energy, and beyond.
- Introduction
- Importance of electrical conductivity in modern technology
- Overview of different types of conductivity (electrical, thermal, etc.)
- Relevance of conductivity in materials science and engineering
- Fundamentals of Conductivity
- Definition and measurement of electrical conductivity
- Relationship between conductivity and resistivity
- Differentiation between conductors, semiconductors, and insulators
- Band theory and conductivity in solids
- Factors Influencing Conductivity
- Impact of temperature on conductivity
- Role of impurities and defects in conductivity
- Crystal structure and its effect on conductivity
- Significance of carrier concentration and mobility
- Applications of Conductivity
- Electrical wiring and power transmission
- Electronic devices and integrated circuits
- Energy storage and conversion systems
- Sensors and detectors
- Biomedical applications
- Emerging technologies (e.g., flexible electronics, wearable devices)
- Challenges in Achieving High Conductivity
- Limitations of conventional conductive materials
- Trade-offs between conductivity and other material properties
- Cost considerations and scalability
- Environmental and sustainability concerns
- Innovations and Strategies for Enhancing Conductivity
- Advanced materials and nanotechnology approaches
- Doping and alloying techniques
- Surface modification and interface engineering
- Molecular and organic conductors
- Conductive polymers and composites
- Two-dimensional materials and graphene
- Case Studies and Success Stories
- Breakthroughs in high-conductivity materials
- Real-world applications and commercialization
- Future Prospects and Outlook
- Emerging trends in conductivity research
- Multidisciplinary collaborations and knowledge exchange
- Potential impact of enhanced conductivity on various industries
- Environmental and sustainable conductivity solutions
- Conclusion
- Summary of key findings and insights
- Importance of conductivity in advancing technology and innovation
- Call for continued research and development in high-conductivity materials
This white paper aims to provide a comprehensive understanding of conductivity, its significance, and the ongoing efforts to enhance conductivity in various fields. By exploring the challenges and advancements in this area, it aims to inspire researchers, scientists, and engineers to further explore the possibilities of high-conductivity materials and their applications.
