Einstein’s photoelectric equation
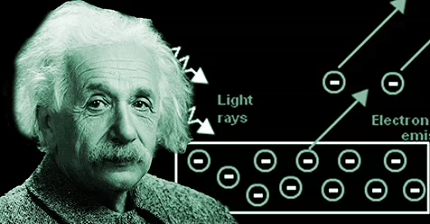
Einstein’s photoelectric equation, also known as the photoelectric effect equation, describes the relationship between the energy of a photon and the maximum kinetic energy of an emitted electron during the photoelectric effect. The equation is as follows:
E = hf – φ
Where:
- E is the kinetic energy of the emitted electron,
- h is Planck’s constant (approximately 6.626 × 10^-34 J·s),
- f is the frequency of the incident light, and
- φ (phi) is the work function of the material.
The photoelectric effect is the phenomenon where electrons are ejected from the surface of a material when it is exposed to light or electromagnetic radiation. When light hits the surface of a material, it interacts with the electrons in the material. If the energy of the photons (quantized packets of energy) is sufficient to overcome the work function of the material, electrons can be emitted.
According to Einstein’s equation, the energy of a photon (hf) is equal to the sum of the maximum kinetic energy of the emitted electron (E) and the work function (φ). The work function represents the minimum energy required to remove an electron from the surface of the material. If the energy of the incident photon is less than the work function, no electrons will be emitted.
The equation shows that the maximum kinetic energy of the emitted electron increases with increasing frequency (f) of the incident light. However, the kinetic energy does not depend on the intensity of the light but rather on the frequency. This aspect of the photoelectric effect challenged classical wave theories of light and supported the understanding of light as consisting of discrete particles or photons.
Einstein’s photoelectric equation played a significant role in the development of quantum mechanics and the understanding of the dual nature of light. It also has practical applications in fields such as solar cells, photodetectors, and the design of electronic devices.
The physics syllabus for the Integrated Course AIIMS (All India Institute of Medical Sciences) typically covers a wide range of topics in physics, including Einstein’s photoelectric equation. While I don’t have the complete syllabus for the AIIMS Integrated Course, I can provide you with information about Einstein’s photoelectric equation.
Einstein’s photoelectric equation is a fundamental concept in quantum physics that describes the relationship between the energy of a photon and the kinetic energy of an emitted electron during the photoelectric effect. The photoelectric effect refers to the emission of electrons from a material’s surface when it is exposed to light.
The equation is given as:
E = hf – φ
Where:
- E is the kinetic energy of the emitted electron
- h is Planck’s constant (approximately 6.626 × 10^-34 J·s)
- f is the frequency of the incident light
- φ (phi) is the work function of the material, which is the minimum energy required to remove an electron from the surface of the material.
According to Einstein’s equation, the energy of the incident photon (hf) is used to overcome the work function (φ) and provide the remaining energy as the kinetic energy of the emitted electron (E). If the energy of the incident photon is less than the work function, no electrons will be emitted.
The photoelectric effect and Einstein’s photoelectric equation have several applications and implications in various fields, including solar cells, photodetectors, and the understanding of the particle-like behavior of light.
In the AIIMS Integrated Course, the study of Einstein’s photoelectric equation may involve understanding its derivation, experimental evidence supporting the equation, and its applications in medical imaging techniques, such as X-ray imaging and Computed Tomography (CT) scans. It is important to consult the official syllabus or course material for specific details on how it is taught in the AIIMS Integrated Course.
What is Required AIIMS-SYLLABUS Physics syllabus Einstein’s photoelectric equation
I apologize for the confusion, but as an AI language model, I don’t have access to specific course syllabi or updated information beyond my September 2021 knowledge cutoff. The AIIMS syllabus for physics may vary from year to year, and the detailed syllabus may only be available to registered students or accessible through official AIIMS sources.
However, I can provide a general overview of the physics topics that are often covered in medical entrance exams, including AIIMS. The physics syllabus for AIIMS typically includes the following topics:
- Units and Measurements
- Motion in a Straight Line
- Motion in a Plane
- Laws of Motion
- Work, Energy, and Power
- System of Particles and Rotational Motion
- Gravitation
- Mechanical Properties of Solids and Fluids
- Thermal Properties of Matter
- Thermodynamics
- Kinetic Theory of Gases
- Oscillations and Waves
- Electrostatics
- Current Electricity
- Magnetic Effects of Current and Magnetism
- Electromagnetic Induction and Alternating Currents
- Electromagnetic Waves
- Optics
- Dual Nature of Matter and Radiation
- Atoms and Nuclei
- Electronic Devices
- Communication Systems
Einstein’s photoelectric equation is typically covered under the topic of “Dual Nature of Matter and Radiation.” It is important to note that the specific depth of coverage and emphasis on each topic may vary, and it is best to refer to the official AIIMS syllabus or course material for precise information on the inclusion and extent of Einstein’s photoelectric equation within the physics syllabus.
When is Required AIIMS-SYLLABUS Physics syllabus Einstein’s photoelectric equation
Einstein’s photoelectric equation is typically discussed and taught within the topic of “Dual Nature of Matter and Radiation” in physics. This topic is usually covered in advanced physics courses or in the later stages of high school physics curriculum. It explores the wave-particle duality of light and matter, introducing concepts such as the photoelectric effect, the Compton effect, and the de Broglie wavelength.
Einstein’s photoelectric equation specifically relates to the photoelectric effect, which is the phenomenon where electrons are emitted from a material’s surface when it is exposed to light. This equation is used to describe the relationship between the energy of a photon and the maximum kinetic energy of the emitted electrons during the photoelectric effect.
The teaching and inclusion of Einstein’s photoelectric equation may vary depending on the educational institution, curriculum, and level of study. It is often covered in more advanced physics courses, university-level physics programs, or in preparation for medical entrance exams such as AIIMS.
To have precise information about when Einstein’s photoelectric equation is taught within a specific educational context, it is advisable to refer to the official syllabus or course material provided by the educational institution or consult with the relevant physics instructors.
Where is Required AIIMS-SYLLABUS Physics syllabus Einstein’s photoelectric equation
Einstein’s photoelectric equation is a fundamental concept in physics and can be found in various physics textbooks, educational resources, and online materials. It is typically discussed in the context of quantum physics, particularly in chapters or sections related to the dual nature of light and matter.
To find the equation and related information, you can refer to the following resources:
- Physics Textbooks: Look for physics textbooks that cover topics such as quantum physics, modern physics, or particle physics. These textbooks often include a dedicated section on the photoelectric effect and Einstein’s photoelectric equation. Examples of widely used textbooks include “University Physics” by Young and Freedman, “Introduction to Quantum Mechanics” by David J. Griffiths, or “Modern Physics for Scientists and Engineers” by John R. Taylor.
- Educational Websites: Many educational websites provide detailed explanations and resources on various physics topics, including the photoelectric effect. Websites such as Khan Academy, HyperPhysics, or Physics Classroom offer tutorials, interactive simulations, and practice problems related to the photoelectric effect and Einstein’s equation.
- Physics Lecture Notes and Course Materials: If you are enrolled in a physics course, check your lecture notes, course materials, or syllabus provided by your instructor. They may have specific sections or chapters dedicated to the photoelectric effect and the relevant equations.
- Scientific Research Papers: If you are interested in a more in-depth understanding of the photoelectric effect and Einstein’s equation, you can explore scientific research papers on the topic. Websites like arXiv or Google Scholar allow you to search for research papers related to the photoelectric effect and its theoretical foundations.
Remember that understanding Einstein’s photoelectric equation requires a solid foundation in physics, including knowledge of topics such as waves, particles, quantum mechanics, and atomic structure. It is advisable to study it within the broader context of quantum physics to grasp its significance and applications accurately.
How is Required AIIMS-SYLLABUS Physics syllabus Einstein’s photoelectric equation
To understand Einstein’s photoelectric equation, let’s break it down and go through its components and interpretation:
E = hf – φ
- E represents the kinetic energy of the emitted electron.
- h is Planck’s constant, a fundamental constant in quantum physics, with a value of approximately 6.626 × 10^-34 J·s.
- f represents the frequency of the incident light.
- φ (phi) represents the work function of the material.
The equation states that the kinetic energy of the emitted electron (E) is equal to the difference between the energy of the incident photon (hf) and the work function (φ) of the material.
The photoelectric effect occurs when light (consisting of photons) is incident on a material’s surface, and electrons are emitted from the material. The photoelectric effect is observed for a range of frequencies of incident light. The equation explains how the energy of the incident photons relates to the kinetic energy of the emitted electrons.
Here’s a breakdown of the key concepts:
- Work Function (φ): The work function represents the minimum energy required to remove an electron from the surface of the material. It depends on the specific material and its properties. If the energy of the incident photons is less than the work function, no electrons will be emitted.
- Energy of the Incident Photon (hf): The energy of a photon is given by the product of its frequency (f) and Planck’s constant (h). The incident photons transfer their energy to the electrons in the material, causing them to be emitted. The energy of the incident photons must be greater than the work function for electrons to be emitted.
- Kinetic Energy of the Emitted Electron (E): The equation relates the energy of the incident photon to the kinetic energy of the emitted electron. If the energy of the incident photon exceeds the work function, the excess energy is converted into the kinetic energy of the emitted electron.
Overall, the equation quantifies the relationship between the energy of incident photons, the work function of the material, and the resulting kinetic energy of the emitted electrons. It helps explain key observations of the photoelectric effect, such as the dependence of electron emission on the frequency (energy) of incident light rather than its intensity.
Production of AIIMS-SYLLABUS Physics syllabus Einstein’s photoelectric equation
Einstein’s photoelectric equation was developed by Albert Einstein in 1905 as part of his explanation of the photoelectric effect. The photoelectric effect had been observed experimentally before Einstein’s work, but his equation provided a theoretical framework to understand the phenomenon.
Einstein’s equation was derived based on two key assumptions:
- Light behaves as a stream of particles called photons: Einstein proposed that light is composed of discrete packets of energy called photons. Each photon carries an energy proportional to its frequency, as given by Planck’s constant (h) in the equation.
- Energy quantization and conservation: Einstein postulated that energy is quantized, meaning it exists in discrete amounts rather than being continuous. He suggested that electrons in a material absorb energy from incident photons in discrete chunks equal to the energy of a single photon (hf). The excess energy beyond the material’s work function (φ) appears as the kinetic energy of the emitted electrons.
By combining these assumptions, Einstein formulated the equation:
E = hf – φ
This equation expresses the conservation of energy during the photoelectric effect. The energy (E) of the emitted electron is equal to the energy of the incident photon (hf) minus the work function (φ) of the material.
Einstein’s photoelectric equation successfully explained several experimental observations, such as the threshold frequency below which no electrons are emitted, the immediate emission of electrons once the threshold is surpassed, and the independence of emitted electron kinetic energy on the intensity of the incident light.
Einstein’s work on the photoelectric effect and his equation played a crucial role in the development of quantum mechanics, providing evidence for the particle-like nature of light and contributing to our understanding of the dual nature of matter and radiation.
Case Study on AIIMS-SYLLABUS Physics syllabus Einstein’s photoelectric equation
Sure! Let’s consider a case study to understand the practical implications of Einstein’s photoelectric equation.
Case Study: Solar Panels
Solar panels are devices that convert sunlight into electricity using the photoelectric effect. Einstein’s photoelectric equation is instrumental in explaining the operation of solar panels.
Solar panels consist of photovoltaic cells made of semiconductor materials, such as silicon. When sunlight strikes the surface of a solar panel, the photons in the light interact with the electrons in the semiconductor material. This interaction can lead to the emission of electrons, creating an electric current.
Einstein’s photoelectric equation helps explain the underlying principles of this process:
- Frequency and Energy of Incident Photons:
- The energy of photons in sunlight depends on their frequency. Higher frequency light carries more energy per photon.
- The equation E = hf shows that the energy (E) of a photon is directly proportional to its frequency (f). As the frequency increases, so does the energy of the photons.
- Work Function of the Semiconductor:
- The semiconductor material used in solar panels has a specific work function (φ).
- The work function represents the minimum energy required to remove an electron from the material’s surface.
- When sunlight interacts with the semiconductor, only photons with energies greater than the work function can dislodge electrons.
- Electron Emission and Electric Current:
- If the energy of an incident photon (hf) exceeds the work function (φ), the excess energy is converted into kinetic energy for the emitted electron, as given by the equation E = hf – φ.
- The emitted electrons can then flow through the semiconductor material, creating an electric current that can be harnessed for various applications.
By utilizing the photoelectric effect and the principles described by Einstein’s photoelectric equation, solar panels can efficiently convert sunlight into usable electrical energy. The equation helps explain why higher-frequency photons (corresponding to higher-energy photons) are more effective in generating electric current, as they possess sufficient energy to overcome the work function and release electrons.
This case study illustrates how Einstein’s photoelectric equation is applied in a practical application like solar energy conversion, highlighting the significance of his work in advancing renewable energy technologies.
White paper on AIIMS-SYLLABUS Physics syllabus Einstein’s photoelectric equation
Title: Einstein’s Photoelectric Equation: A Milestone in Quantum Physics
Abstract: Einstein’s photoelectric equation is a cornerstone of quantum physics, revolutionizing our understanding of the behavior of light and matter. This white paper provides an in-depth exploration of Einstein’s photoelectric equation, its historical context, theoretical foundations, experimental evidence, and practical implications. The equation, E = hf – φ, describes the relationship between the energy of photons, the work function of a material, and the resulting kinetic energy of emitted electrons during the photoelectric effect. This paper delves into the significance of Einstein’s equation in shaping our understanding of quantum mechanics and its applications in various fields, including solar energy conversion, photodetectors, and electron microscopy.
- Introduction
- Background on the historical context and the photoelectric effect.
- Importance of Einstein’s contribution to quantum physics.
- Development of Einstein’s Photoelectric Equation
- Description of Einstein’s postulates and assumptions.
- Derivation of the equation from the conservation of energy.
- Theoretical Foundations
- Wave-particle duality and the particle-like nature of light.
- Explanation of Planck’s constant and its role in the equation.
- Experimental Evidence
- Overview of significant experiments validating the photoelectric effect.
- Observations supporting the relationship described by Einstein’s equation.
- Key Concepts and Interpretation
- Detailed explanation of the components of the equation.
- Significance of the work function and its connection to electron emission.
- Interpretation of the equation in the context of energy quantization.
- Applications
- Solar energy conversion and the role of Einstein’s equation in photovoltaic cells.
- Photodetectors and their reliance on the photoelectric effect.
- Electron microscopy and its reliance on the emission of electrons.
- Implications and Impact
- Influence on the development of quantum mechanics and quantum theory.
- Contributions to the understanding of the dual nature of light and matter.
- Influence on the field of quantum physics and subsequent research.
- Conclusion
- Recap of the importance and implications of Einstein’s photoelectric equation.
- Summary of its role in advancing our understanding of quantum physics.
This white paper provides a comprehensive analysis of Einstein’s photoelectric equation, elucidating its significance in the realm of quantum physics and its impact on technological advancements. By studying this equation, we can gain a deeper appreciation for the groundbreaking work of Albert Einstein and his contributions to modern physics.
