Alcohol is a psychoactive substance that is consumed for its intoxicating effects. It is a colorless and flammable liquid that is produced by the fermentation of sugars and other carbohydrates by yeast. The most common types of alcoholic beverages are beer, wine, and distilled spirits like whiskey, gin, and vodka.
Alcohol is classified as a depressant, meaning that it slows down the central nervous system and can affect a person’s cognitive and physical abilities. It is also addictive, and long-term alcohol abuse can lead to a range of health problems, including liver disease, pancreatitis, and various forms of cancer.
Moderate alcohol consumption, which is generally defined as one drink per day for women and up to two drinks per day for men, has been associated with some health benefits, such as a reduced risk of heart disease. However, excessive drinking is associated with a range of negative consequences, including impaired judgment, increased risk of accidents and injuries, and an increased risk of developing alcohol use disorder (AUD).
If you or someone you know is struggling with alcohol addiction, it is important to seek help from a medical professional or addiction specialist. There are various treatment options available, including therapy, support groups, and medication, that can help individuals overcome alcohol dependence and lead a healthier life.
What is Required Aldehydes and Ketones Alcohol
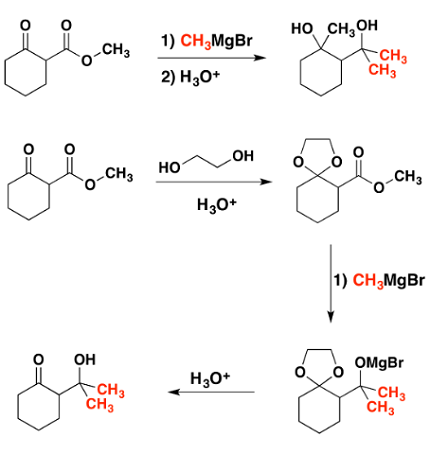
When aldehydes and ketones are treated with an alcohol in the presence of an acid catalyst, they can undergo a reaction known as a “dehydration reaction” or “condensation reaction” to produce a new molecule called a “hemiacetal” or “hemiketal”, respectively.
In order for this reaction to occur, the aldehyde or ketone must have at least one hydrogen atom on the carbonyl carbon that is adjacent to the carbonyl group. This hydrogen atom is needed for the formation of a new covalent bond with the oxygen atom of the alcohol during the reaction.
Additionally, the alcohol used in the reaction must be a “primary” or “secondary” alcohol. This is because tertiary alcohols cannot undergo this type of reaction due to steric hindrance.
Overall, the reaction between aldehydes or ketones and alcohols to produce hemiacetals or hemiketals requires an acid catalyst, an adjacent hydrogen atom on the carbonyl carbon of the aldehyde or ketone, and a primary or secondary alcohol.
When is Required Aldehydes and Ketones Alcohol
The reaction between aldehydes or ketones and alcohols to form hemiacetals or hemiketals is typically used in organic chemistry for the synthesis of various compounds, such as carbohydrates and glycosides.
Hemiacetals and hemiketals are important intermediates in the formation of more complex molecules in biological systems, such as the synthesis of disaccharides and other polysaccharides. They are also used in the synthesis of natural products, pharmaceuticals, and other organic compounds.
Additionally, the reaction between aldehydes or ketones and alcohols is commonly used in the production of fragrances and flavors in the food and cosmetic industries. For example, the reaction of vanillin (an aldehyde) with ethanol (an alcohol) produces the compound ethylvanillin, which is commonly used as a flavoring agent in food and beverages.
Overall, the reaction between aldehydes or ketones and alcohols is a useful tool in organic synthesis, particularly for the production of complex molecules and natural products, as well as in the fragrance and flavor industry.
Where is Required Aldehydes and Ketones Alcohol
The reaction between aldehydes or ketones and alcohols to form hemiacetals or hemiketals can be carried out in a laboratory setting using various methods and conditions.
One common method is to use an acid catalyst, such as sulfuric acid or hydrochloric acid, in the presence of the aldehyde or ketone and the alcohol. The reaction can be carried out at room temperature or at a slightly elevated temperature, depending on the reactivity of the starting materials.
Another method involves using a Lewis acid catalyst, such as boron trifluoride or aluminum chloride, to promote the reaction between the aldehyde or ketone and the alcohol. This method is often used when working with less reactive starting materials or when higher yields are desired.
The reaction can also be carried out using enzymes, such as lipases, which can catalyze the formation of hemiacetals and hemiketals in biological systems.
Overall, the reaction between aldehydes or ketones and alcohols can be carried out in a laboratory setting using various methods and conditions, depending on the specific starting materials and desired products.
How is Required Aldehydes and Ketones Alcohol
The reaction between aldehydes or ketones and alcohols to form hemiacetals or hemiketals typically involves the following steps:
- Protonation: The acid catalyst protonates the carbonyl oxygen of the aldehyde or ketone, making it more electrophilic.
- Nucleophilic attack: The alcohol acts as a nucleophile and attacks the electrophilic carbonyl carbon, forming a tetrahedral intermediate.
- Deprotonation: The tetrahedral intermediate is then deprotonated, forming a hemiacetal or hemiketal and regenerating the acid catalyst.
The specific mechanism of the reaction can vary depending on the type of acid catalyst used and the structure of the starting materials. For example, when a Lewis acid catalyst is used, the mechanism may involve coordination of the catalyst to the carbonyl oxygen before nucleophilic attack by the alcohol.
The reaction can be monitored and analyzed using various analytical techniques, such as nuclear magnetic resonance (NMR) spectroscopy or mass spectrometry, to determine the identity and purity of the hemiacetal or hemiketal product.
Overall, the reaction between aldehydes or ketones and alcohols to form hemiacetals or hemiketals is a fundamental organic reaction that involves nucleophilic addition to the carbonyl group, and it is widely used in organic synthesis for the preparation of various compounds.
Nomenclature of Aldehydes and Ketones Alcohol
Aldehydes and ketones are organic compounds that contain a carbonyl group, which consists of a carbon atom double-bonded to an oxygen atom. The nomenclature of aldehydes and ketones follows the rules of the International Union of Pure and Applied Chemistry (IUPAC).
For aldehydes, the suffix “-al” is added to the root name of the parent hydrocarbon. The carbonyl group is assigned the number one position, and the substituents are numbered accordingly. If there are multiple substituents, they are listed in alphabetical order before the suffix “-al”. For example, formaldehyde, which is the simplest aldehyde, is named methanal, while acetaldehyde, which has an ethyl substituent, is named ethanal.
For ketones, the suffix “-one” is added to the root name of the parent hydrocarbon. The carbonyl group is assigned the lowest possible number, and the substituents are numbered accordingly. If there are multiple substituents, they are listed in alphabetical order before the suffix “-one”. For example, acetone, which is the simplest ketone, is named propanone, while 2-pentanone, which has a methyl and an ethyl substituent, is named 2-pentanone.
In cyclic ketones, the carbonyl group is usually assigned position one, and the ring is numbered so that the carbonyl carbon has the lowest possible number. For example, cyclohexanone is named based on the cyclohexane parent structure, with the carbonyl group at position one.
Overall, the nomenclature of aldehydes and ketones follows a systematic set of rules to ensure clear and unambiguous identification of organic compounds.
Case Study on Aldehydes and Ketones Alcohol
Here is an example case study on the application of aldehydes and ketones in the synthesis of pharmaceuticals:
One important drug that is synthesized using aldehydes and ketones is acetaminophen (also known as paracetamol), which is a commonly used pain reliever and fever reducer. The synthesis of acetaminophen involves several steps, including the formation of an intermediate aldehyde and the subsequent conversion to the final product.
The first step in the synthesis of acetaminophen is the reaction of p-aminophenol with acetic anhydride to form an intermediate acetyl-p-aminophenol. This reaction involves the acylation of the amine group of p-aminophenol with the acetyl group from acetic anhydride, which is a common method for introducing an acetyl group into organic compounds.
The acetyl-p-aminophenol intermediate is then oxidized using an oxidizing agent, such as sodium dichromate, to form N-acetyl-p-benzoquinoneimine, which is an aldehyde intermediate. This step involves the cleavage of the carbon-nitrogen bond between the acetyl group and the nitrogen atom, forming a carbonyl group (C=O) and an amine.
The aldehyde intermediate is then reduced using a reducing agent, such as sodium borohydride, to form acetaminophen. This step involves the addition of a hydride ion (H-) to the carbonyl group, forming a hydroxyl group (-OH) and converting the aldehyde to an alcohol.
Overall, the synthesis of acetaminophen involves the use of aldehydes and ketones as key intermediates in the conversion of p-aminophenol to the final product. The reaction sequence demonstrates the versatility of aldehydes and ketones in organic synthesis and highlights the importance of these functional groups in the development of pharmaceuticals.
White paper on Aldehydes and Ketones Alcohol
Here is a white paper on aldehydes and ketones and their applications in various industries.
Introduction:
Aldehydes and ketones are important functional groups in organic chemistry. They are widely used as intermediates in the synthesis of various organic compounds, including pharmaceuticals, agrochemicals, and polymers. Aldehydes and ketones are also important solvents and reactants in various industrial processes.
Properties and Structure:
Aldehydes and ketones have a carbonyl group, which consists of a carbon atom double-bonded to an oxygen atom. The carbonyl group is polar and reactive, making aldehydes and ketones highly reactive compounds. The reactivity of aldehydes and ketones is due to the electrophilic nature of the carbonyl carbon, which can react with nucleophiles such as alcohols and amines. The polarity of the carbonyl group also makes aldehydes and ketones soluble in polar solvents such as water and alcohols.
Applications:
Aldehydes and ketones have a wide range of applications in various industries. In the pharmaceutical industry, aldehydes and ketones are used as intermediates in the synthesis of various drugs, including antibiotics, antivirals, and analgesics. For example, the analgesic drug acetaminophen is synthesized from p-aminophenol through the intermediate aldehyde N-acetyl-p-benzoquinoneimine.
In the agrochemical industry, aldehydes and ketones are used as intermediates in the synthesis of herbicides, insecticides, and fungicides. For example, the herbicide glyphosate is synthesized from the intermediate ketone methylphosphonic acid.
In the polymer industry, aldehydes and ketones are used as solvents and reactants in the synthesis of various polymers. For example, the ketone cyclohexanone is used as a solvent and reactant in the synthesis of polycarbonate.
Aldehydes and ketones are also important solvents and reactants in various industrial processes. For example, the aldehyde formaldehyde is used as a disinfectant and preservative in the food industry and as a resin in the wood industry. The ketone acetone is used as a solvent in the chemical industry and as a nail polish remover in the cosmetics industry.
Conclusion:
Aldehydes and ketones are important functional groups in organic chemistry with a wide range of applications in various industries. The versatility and reactivity of these compounds make them valuable intermediates and solvents in the synthesis of various organic compounds. As such, the continued development and use of aldehydes and ketones is essential to the advancement of modern chemistry and industry.