Boron is a unique element with several anomalous properties, some of which include:
- Low melting and boiling point: Boron has a very high melting and boiling point for a non-metal, but its values are still relatively low compared to other elements with similar properties.
- Hardness: Boron is one of the hardest elements known, with a Mohs hardness of 9.3. This makes it an excellent material for use in armor and cutting tools.
- Electron deficiency: Boron has only three valence electrons, which is one less than the typical number of valence electrons for elements in the same period. This electron deficiency gives boron unique chemical properties, including its ability to form strong covalent bonds with other elements.
- Unusual bonding: Boron’s electron deficiency also causes it to form unusual bonding structures. For example, it can form planar, three-coordinate molecules with a central boron atom and three terminal atoms or ligands. These structures are known as boranes.
- Isotope abundance: The isotopes of boron have an unusual distribution, with two stable isotopes, boron-10 and boron-11, in roughly equal abundance. This isotopic anomaly is believed to be due to the way boron was formed in the universe.
Overall, these anomalous properties of boron make it a fascinating element with unique chemical and physical properties that have important practical applications in various fields.
Boron group
The boron bunch are the compound components in bunch 13 of the occasional table, containing boron (B), aluminum (Al), gallium (Ga), indium (In), thallium (Tl), and nihonium (Nh). The components in the boron bunch are portrayed by having three valence electrons. These components have additionally been alluded to as the triels.
Boron is regularly named a (metalloid) while the rest, with the conceivable exemption of nihonium, are viewed as post-change metals. Boron happens meagerly, likely in light of the fact that siege by the subatomic particles delivered from regular radioactivity upsets its cores. Aluminum happens generally on the planet, and for sure is the third most bountiful component in the World’s outside layer (8.3%). Gallium is found in the earth with an overflow of 13 ppm. Indium is the 61st most bountiful component in the world’s covering, and thallium is tracked down in moderate sums all through the planet. Nihonium isn’t known to happen in nature and in this way is named an engineered component.
A few gathering 13 components play organic parts in the environment. Boron is a minor component in people and is fundamental for certain plants. Absence of boron can prompt hindered plant development, while an overabundance can likewise hurt by restraining development. Aluminum plays neither an organic part nor huge harmfulness and is viewed as protected. Indium and gallium can invigorate digestion; gallium is attributed with the capacity to tie itself to press proteins. Thallium is profoundly harmful, obstructing the capability of various indispensable compounds, and has considered use to be a pesticide.
Boron arsenide
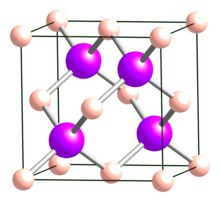
Boron arsenide (or Arsenic boride) is a substance compound including boron and arsenic, normally with a synthetic recipe BAs. Other boron arsenide compounds are referred to, for example, the subarsenide B12As2. Synthetic union of cubic BAs is extremely difficult and its single precious stone structures typically have deserts.
Boron subarsenide
Boron arsenide likewise happens as subarsenides, including the icosahedral boride B12As2. It has a place with R3m space bunch with a rhombohedral construction in view of groups of boron iotas and two-molecule As chains. It is a wide-bandgap semiconductor (3.47 eV) with the remarkable capacity to “self-recuperate” radiation harm. This structure can be developed on substrates like silicon carbide. One more use for sun oriented cell manufacture was proposed, however it isn’t as of now utilized for this reason.
Production of Anomalous properties of boron
Boron is produced industrially through a few different processes. The most common method is the reduction of boron oxide (B2O3) with magnesium (Mg) metal. This reaction takes place at high temperatures (around 1000°C) in an inert atmosphere, typically using an electric furnace. The resulting product is a mixture of boron and magnesium oxide, which can be separated through a series of chemical and physical processes.
Another method for producing boron is through the reaction of boron trichloride (BCl3) with hydrogen gas (H2) in the presence of a catalyst. This process, known as the Bergius process, can be used to produce high-purity boron with a low impurity content.
There are also several other methods for producing boron, including the carbothermic reduction of boron oxide with carbon, the electrolysis of molten borate salts, and the reduction of boron halides with alkali metals.
Once produced, boron can be purified through a series of chemical and physical processes to remove impurities and obtain high-purity boron. Purification methods can include sublimation, distillation, and chemical treatments.
The anomalous properties of boron, such as its hardness, high cross-section for absorbing thermal neutrons, and unique bonding behavior, make it useful in various applications, including nuclear reactors, cutting tools, and refractory materials. The production of boron is an important industrial process that enables these practical applications of this fascinating element.
How is boron
Boron is a chemical element with the symbol B and atomic number 5. It is a metalloid, which means it has properties of both metals and nonmetals. Boron is a relatively rare element in the Earth’s crust, making up only about 0.001% of the crust by weight.
In terms of its physical properties, boron is a hard, brittle material that is black or dark brown in color. It has a high melting point and is a poor conductor of electricity at room temperature. Boron is also known for its ability to absorb neutrons, which makes it useful in nuclear applications.
In terms of its chemical properties, boron is reactive and forms compounds with many other elements. It is commonly found in nature in the form of borates, which are salts that contain boron and oxygen. Boron has a variety of industrial and technological applications, including in the production of fiberglass, ceramics, and semiconductors. It is also used in agriculture as a micronutrient for plants.
Case Study on Anomalous properties of boron
One interesting case study involving the anomalous properties of boron is its use in nuclear reactors. Boron is a key material used in the control rods of nuclear reactors, which are used to regulate the rate of fission reactions in the reactor core.
Boron’s unique properties make it an ideal material for this application. Specifically, boron has a very high cross-section for absorbing thermal neutrons, which are the particles responsible for initiating fission reactions in a nuclear reactor. When boron absorbs a neutron, it forms a short-lived compound known as boron-11, which quickly decays into a stable compound, releasing energy in the process.
Control rods made of boron are typically made from a boron-carbide composite material, which combines boron with carbon to create a material that is both strong and resistant to corrosion. These control rods are inserted into the reactor core during operation to absorb neutrons and slow down the rate of fission reactions.
The anomalous properties of boron also come into play in the design of nuclear reactors. For example, the unusual bonding behavior of boron allows it to form complex compounds with other elements, which can be used as neutron-absorbing materials in nuclear fuels.
Overall, the anomalous properties of boron make it an important material for nuclear applications, where its ability to absorb neutrons and control nuclear reactions is critical for the safe and efficient operation of nuclear power plants.
White paper on Anomalous properties of boron
Here is a white paper on the anomalous properties of boron:
Introduction
Boron is a unique element with several anomalous properties that make it a fascinating subject of study for chemists and physicists alike. Its unique properties stem from its electron configuration, which includes only three valence electrons and an electron deficiency that causes it to form unusual bonding structures. In this white paper, we will explore the anomalous properties of boron and their practical applications.
Electron Deficiency
Boron has only three valence electrons, which is one less than the typical number of valence electrons for elements in the same period. This electron deficiency gives boron unique chemical properties, including its ability to form strong covalent bonds with other elements. This is because boron has an empty p-orbital in its outermost shell, which allows it to form three covalent bonds with other elements. This results in an unusual bonding behavior that is not seen in other elements.
Unusual Bonding
Boron’s electron deficiency causes it to form unusual bonding structures. For example, it can form planar, three-coordinate molecules with a central boron atom and three terminal atoms or ligands. These structures are known as boranes. Boranes are highly reactive and can be used as reducing agents in organic chemistry. They can also be used as precursors to other boron compounds, such as boron carbides.
Hardness
Boron is one of the hardest elements known, with a Mohs hardness of 9.3. This makes it an excellent material for use in armor and cutting tools. Boron carbide, in particular, is a common material used in bulletproof vests and body armor. Boron’s hardness also makes it useful as an abrasive material in industrial applications.
Isotope Abundance
The isotopes of boron have an unusual distribution, with two stable isotopes, boron-10 and boron-11, in roughly equal abundance. This isotopic anomaly is believed to be due to the way boron was formed in the universe. Boron-10 has a very high cross-section for absorbing thermal neutrons, which makes it useful in nuclear applications. Boron-11 is used in nuclear magnetic resonance spectroscopy (NMR) and other analytical techniques.
Low Melting and Boiling Point
Boron has a very high melting and boiling point for a non-metal, but its values are still relatively low compared to other elements with similar properties. Boron has a melting point of 2349°C and a boiling point of 4200°C. This makes it useful in high-temperature applications, such as in refractory materials and in rocket nozzles.
Conclusion
Boron’s anomalous properties make it a fascinating element with unique chemical and physical properties that have important practical applications in various fields. Its ability to form unusual bonding structures, its hardness, and its high cross-section for absorbing thermal neutrons make it useful in nuclear applications. Its unique properties have also led to its use in armor, cutting tools, refractory materials, and rocket nozzles. Boron is a versatile material with a wide range of applications, and its anomalous properties continue to be the subject of study by scientists and researchers.
