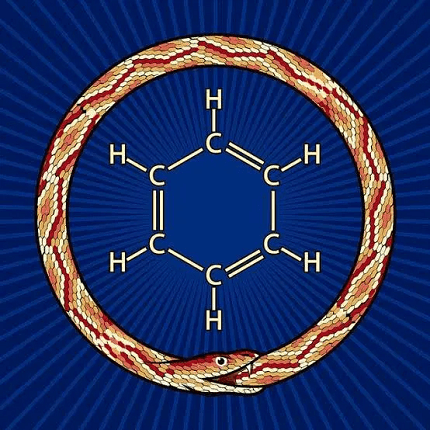
Aromaticity is a chemical property that describes the special stability of certain cyclic molecules, known as aromatic compounds, that contain a specific arrangement of alternating double bonds (pi bonds) and single bonds (sigma bonds). This arrangement of bonds results in a delocalized pi-electron system that is particularly stable and resistant to reactions that would normally break apart double bonds.
The concept of aromaticity was first introduced by German chemist Friedrich August Kekulé in 1865, who proposed that benzene, a cyclic hydrocarbon with the molecular formula C6H6, was made up of six carbon atoms joined in a hexagonal ring with alternating double bonds.
Aromatic compounds have unique physical and chemical properties that set them apart from other compounds. For example, they often have characteristic odors and colors, and they tend to be more stable and less reactive than non-aromatic compounds. Aromatic compounds also play important roles in biological systems, such as in the structure of DNA and many neurotransmitters.
The rules for determining whether a molecule is aromatic are based on the number of electrons in the pi-electron system and the symmetry of the molecule. According to the Hückel rule, a cyclic molecule is considered aromatic if it has a continuous ring of p-orbitals with 4n+2 pi electrons, where n is an integer. Aromaticity can also be influenced by substituents on the ring, with some groups increasing or decreasing the molecule’s aromatic character.
What is Required Basic Principles of Organic Chemistry Aromaticity
There are several principles of organic chemistry that are required to understand aromaticity:
- Resonance: Aromaticity is a consequence of resonance stabilization. In an aromatic system, the pi-electrons are delocalized over the entire ring, resulting in increased stability due to resonance.
- Planarity: In order for a molecule to be aromatic, it must be planar. This is because the pi-electrons must be able to be delocalized around the ring, which requires the molecule to be flat.
- Huckel’s rule: According to Huckel’s rule, a compound is aromatic if it has a cyclic arrangement of pi-electrons with (4n+2) pi-electrons, where n is an integer. This rule explains why some cyclic compounds are aromatic, while others are not.
- Ring size: The ring size of an aromatic compound is important. In general, rings with 6 pi-electrons (such as benzene) are more stable than other ring sizes, although there are some exceptions.
- Substituents: The presence of substituents on an aromatic ring can affect its aromaticity. Some substituents can increase the stability of the ring, while others can decrease it. For example, an electron-donating substituent such as -CH3 can increase the stability of an aromatic ring, while an electron-withdrawing substituent such as -NO2 can decrease it.
- Aromaticity vs. antiaromaticity: Aromaticity is a stabilizing factor, while antiaromaticity is a destabilizing factor. A compound is antiaromatic if it has a cyclic arrangement of pi-electrons with 4n pi-electrons, where n is an integer. Anti-aromatic compounds are generally less stable and more reactive than non-aromatic or aromatic compounds.
When is Required Basic Principles of Organic Chemistry Aromaticity
A knowledge of the basic principles of organic chemistry and aromaticity is required in many areas of chemistry, including:
- Organic synthesis: Understanding aromaticity is important in organic synthesis, as many reactions involve aromatic compounds. For example, the synthesis of drugs, natural products, and polymers often involves the use of aromatic compounds.
- Medicinal chemistry: Many drugs and drug candidates contain aromatic rings, and their biological activity is often related to the aromaticity of the molecule.
- Materials science: Aromatic compounds are used in the production of a wide range of materials, such as plastics, fibers, and coatings. Understanding the aromaticity of these compounds can help in the design and synthesis of new materials.
- Physical organic chemistry: The study of the physical properties of aromatic compounds is an important area of physical organic chemistry. Understanding the electronic structure and bonding in these compounds can help to explain their reactivity and spectroscopic properties.
- Biochemistry: Aromatic compounds are important in biological systems, such as in the structure of DNA and many neurotransmitters. Understanding the aromaticity of these compounds can help in the study of their biological function.
Overall, a knowledge of the basic principles of organic chemistry and aromaticity is important in many areas of chemistry, and is essential for a thorough understanding of organic chemistry as a whole.
Where is Required Basic Principles of Organic Chemistry Aromaticity
A knowledge of the basic principles of organic chemistry and aromaticity is required in various fields of science and technology. Some of the places where this knowledge is required are:
- Organic chemistry laboratories: Organic chemistry laboratories involve the synthesis and analysis of organic compounds, many of which are aromatic. A thorough understanding of the basic principles of organic chemistry and aromaticity is essential for the successful synthesis and characterization of these compounds.
- Pharmaceutical industry: Many drugs and drug candidates contain aromatic rings, and understanding the aromaticity of these compounds is important in drug design and discovery.
- Materials science: Aromatic compounds are used in the production of a wide range of materials, such as plastics, fibers, and coatings. A thorough understanding of the aromaticity of these compounds is essential in the design and synthesis of new materials.
- Petrochemical industry: The petrochemical industry involves the production of chemicals and materials from petroleum and natural gas. Many of these compounds are aromatic, and a thorough understanding of the basic principles of organic chemistry and aromaticity is required for the synthesis and characterization of these compounds.
- Environmental science: Aromatic compounds are common environmental pollutants, and understanding their chemical properties and reactivity is important for their detection, remediation, and prevention.
Overall, a knowledge of the basic principles of organic chemistry and aromaticity is essential in many areas of science and technology, and is important for the successful synthesis, characterization, and application of organic compounds.
How is Required Basic Principles of Organic Chemistry Aromaticity
The basic principles of organic chemistry and aromaticity are usually taught in undergraduate and graduate level chemistry courses. These courses may include:
- Organic Chemistry I and II: These courses cover the fundamentals of organic chemistry, including bonding, stereochemistry, reaction mechanisms, and functional groups. The concept of aromaticity is often introduced in Organic Chemistry II.
- Physical Organic Chemistry: This course covers the physical properties of organic compounds, including spectroscopy, thermodynamics, and kinetics. The concept of aromaticity is often discussed in the context of electronic structure and bonding.
- Medicinal Chemistry: This course focuses on the design and synthesis of drugs, including the role of aromatic compounds in drug design.
- Polymer Chemistry: This course covers the synthesis and properties of polymers, many of which are based on aromatic compounds.
In addition to these courses, there are also many textbooks and online resources available that cover the basic principles of organic chemistry and aromaticity. Students can also supplement their learning by participating in research projects, attending seminars and conferences, and working in chemistry labs.
Overall, a thorough understanding of the basic principles of organic chemistry and aromaticity is essential for success in many areas of science and technology, and can be acquired through coursework, research, and self-study.
Production of Basic Principles of Organic Chemistry Aromaticity
The basic principles of organic chemistry and aromaticity are not “produced” as such, but rather taught and learned through various means. Here are some ways in which these principles can be disseminated and taught:
- University courses: The basic principles of organic chemistry and aromaticity are typically taught in undergraduate and graduate-level chemistry courses. These courses are typically offered by universities and cover the fundamental concepts and theories of organic chemistry and aromaticity.
- Textbooks: There are numerous textbooks available that cover the basic principles of organic chemistry and aromaticity. These books are widely used in university courses and provide a comprehensive overview of the subject matter.
- Online resources: There are numerous online resources available that cover the basic principles of organic chemistry and aromaticity. These resources include online courses, video lectures, interactive tutorials, and online communities.
- Research projects: Students can also learn about organic chemistry and aromaticity through participating in research projects. This provides an opportunity to apply the theoretical concepts learned in courses to practical problems and gain hands-on experience in the field.
- Chemistry labs: Chemistry labs provide an opportunity for students to conduct experiments and gain practical experience in organic chemistry. Students can apply the theoretical concepts learned in courses to real-world problems and gain a deeper understanding of the subject matter.
Overall, the basic principles of organic chemistry and aromaticity can be learned through a combination of coursework, textbooks, online resources, research projects, and practical experience in chemistry labs.
Case Study on Basic Principles of Organic Chemistry Aromaticity
Here is a case study that illustrates the importance of the basic principles of organic chemistry and aromaticity:
Case Study: Designing an Aromatic Molecule for Drug Development
In the field of drug development, the design of new molecules with desired pharmacological properties is a critical step. Aromatic compounds are commonly used in drug design, as they can interact with specific receptors in the body and are often more stable and bioavailable than non-aromatic compounds.
In this case study, a pharmaceutical company is developing a new drug to treat a specific disease. The company’s chemists are tasked with designing an aromatic molecule that can interact with a specific receptor in the body and exhibit the desired pharmacological properties.
The chemists start by considering the basic principles of organic chemistry and aromaticity. They know that aromatic compounds are characterized by a cyclic, planar structure, with a delocalized pi electron system. They also know that the number of pi electrons in the aromatic ring is critical to its stability and reactivity, as well as its ability to interact with specific receptors in the body.
Using this knowledge, the chemists design a molecule that contains an aromatic ring with the correct number of pi electrons to interact with the receptor of interest. They also carefully choose the substituents on the ring to optimize the molecule’s pharmacological properties, such as its solubility, bioavailability, and potency.
After synthesizing the molecule and conducting in vitro and in vivo studies, the company’s scientists find that the molecule exhibits the desired pharmacological properties and has potential for further development as a drug.
This case study illustrates how a knowledge of the basic principles of organic chemistry and aromaticity is essential in the design and development of new drugs. Without a thorough understanding of these concepts, the chemists would not have been able to design an aromatic molecule with the desired pharmacological properties, and the drug development process would have been much more difficult and uncertain.
White paper on Basic Principles of Organic Chemistry Aromaticity
Introduction
The concept of aromaticity is a fundamental principle in organic chemistry. Aromatic compounds are characterized by a cyclic, planar structure with a delocalized pi electron system. These compounds are often more stable and reactive than non-aromatic compounds and have a wide range of applications in organic synthesis, materials science, and drug design.
This white paper provides an overview of the basic principles of organic chemistry and aromaticity, including the history of the concept, the electronic structure of aromatic compounds, and their properties and applications.
History of Aromaticity
The concept of aromaticity was first introduced in the late 19th century by the German chemist August Kekulé. Kekulé proposed that certain cyclic compounds, such as benzene, contained alternating double bonds and were characterized by a stable, delocalized pi electron system.
The term “aromatic” was later introduced by the German chemist Carl Graebe to describe these compounds, as many of them had a pleasant, fragrant odor. Over time, the definition of aromaticity was refined to include compounds with a cyclic, planar structure and a delocalized pi electron system that obeys Hückel’s rule.
Electronic Structure of Aromatic Compounds
The electronic structure of aromatic compounds is characterized by a cyclic, planar structure with a delocalized pi electron system. The delocalization of the pi electrons is responsible for the stability and reactivity of these compounds, as it allows them to participate in a variety of chemical reactions.
Aromatic compounds obey Hückel’s rule, which states that a compound is aromatic if it contains a cyclic, planar structure with 4n+2 pi electrons, where n is an integer. This rule explains why many aromatic compounds contain 6 pi electrons, as in the case of benzene.
Properties and Applications of Aromatic Compounds
Aromatic compounds have a wide range of properties and applications in organic chemistry. They are often more stable and reactive than non-aromatic compounds, and are commonly used in organic synthesis, materials science, and drug design.
In organic synthesis, aromatic compounds are used as starting materials or intermediates in a variety of reactions, such as electrophilic aromatic substitution and nucleophilic aromatic substitution. Aromatic compounds are also used in materials science to create polymers with desirable properties, such as high strength and flexibility.
In drug design, aromatic compounds are often used as scaffolds to create molecules with specific pharmacological properties. The cyclic, planar structure of aromatic compounds allows them to interact with specific receptors in the body, making them valuable in the development of new drugs.
Conclusion
The basic principles of organic chemistry and aromaticity are essential for understanding the properties and applications of aromatic compounds. Aromatic compounds are characterized by a cyclic, planar structure with a delocalized pi electron system, and are often more stable and reactive than non-aromatic compounds. These compounds have a wide range of applications in organic synthesis, materials science, and drug design, making them an important area of study in organic chemistry.
