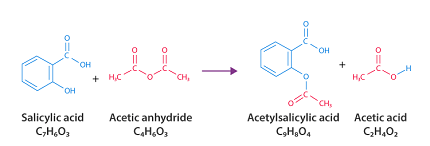
Aspirin, also known as acetylsalicylic acid, can be synthesized from salicylic acid and acetic anhydride. Here are the steps for the synthesis of aspirin:
Materials:
- Salicylic acid
- Acetic anhydride
- Sulfuric acid (catalyst)
- Water
- Sodium bicarbonate (baking soda)
- Ethanol
Equipment:
- Beaker
- Erlenmeyer flask
- Glass stirring rod
- Bunsen burner or hot plate
- Thermometer
- Filter paper
- Funnel
- Buchner funnel
- Vacuum pump
Steps:
- In a beaker, measure 5 g of salicylic acid and add 10 mL of acetic anhydride.
- Add 5 drops of sulfuric acid catalyst to the mixture and stir with a glass rod.
- Heat the mixture on a Bunsen burner or hot plate until the temperature reaches 80-85°C. Keep stirring.
- After 10-15 minutes, remove the beaker from the heat and let it cool to room temperature.
- Slowly add 20 mL of cold water to the mixture, stirring continuously.
- Add a small amount of sodium bicarbonate (baking soda) to the mixture until it is slightly basic (pH around 7-8).
- Filter the mixture through filter paper and collect the solid aspirin product.
- Rinse the aspirin with cold water to remove any impurities.
- Dry the aspirin by placing it on a watch glass in a warm place, or by using an oven or desiccator.
- Weigh the aspirin product and calculate the percent yield.
- Recrystallize the aspirin in ethanol to obtain a purer product, if desired.
Note: Aspirin synthesis involves the use of hazardous chemicals and should only be performed under appropriate laboratory conditions with proper safety precautions.
What is Required Phenols Aspirin synthesis
To synthesize aspirin from phenols, the following reagents and equipment are required:
Reagents:
- Phenol
- Acetic anhydride
- Sulfuric acid (catalyst)
- Water
- Sodium bicarbonate (baking soda)
- Ethanol
Equipment:
- Beaker
- Erlenmeyer flask
- Glass stirring rod
- Bunsen burner or hot plate
- Thermometer
- Filter paper
- Funnel
- Buchner funnel
- Vacuum pump
Note: It is important to use appropriate laboratory safety measures, including gloves, goggles, and a lab coat when working with these chemicals. Also, it is recommended to work in a fume hood or a well-ventilated area.
When is Required Phenols Aspirin synthesis
Phenols can be used in the synthesis of aspirin when salicylic acid is not available or when it is difficult to obtain. The use of phenols as starting material in aspirin synthesis can be advantageous as phenols are more readily available and can be converted into aspirin in fewer steps compared to salicylic acid.
Additionally, using phenols can lead to a higher yield of aspirin as the reaction of phenol with acetic anhydride is more efficient than that of salicylic acid.
However, it is important to note that the synthesis of aspirin from phenols requires the use of hazardous chemicals and should only be performed under appropriate laboratory conditions with proper safety precautions.
Where is Required Phenols Aspirin synthesis
The synthesis of aspirin from phenols can be performed in a laboratory setting, typically in a chemistry lab equipped with the necessary reagents, equipment, and safety measures.
This synthesis can also be performed in a pharmaceutical or industrial setting for the large-scale production of aspirin.
It is important to note that the use of hazardous chemicals in this synthesis requires careful attention to safety and proper disposal of waste materials. Therefore, it should only be performed by trained professionals in a controlled environment.
How is Required Phenols Aspirin synthesis
The synthesis of aspirin from phenols involves the following steps:
- Preparation of the reaction mixture: In a beaker, phenol is mixed with acetic anhydride and a small amount of sulfuric acid catalyst is added. The mixture is then stirred with a glass rod.
- Heating: The beaker is heated on a Bunsen burner or hot plate to a temperature of 50-55°C, and the mixture is stirred continuously.
- Cooling: After 5-10 minutes of heating, the beaker is removed from the heat and allowed to cool to room temperature.
- Hydrolysis: Cold water is slowly added to the cooled mixture, while stirring continuously. This hydrolyzes the excess acetic anhydride to acetic acid and also separates the aspirin product from the reaction mixture.
- Neutralization: A small amount of sodium bicarbonate (baking soda) is added to the mixture to adjust the pH to around 7-8.
- Filtration: The mixture is filtered through filter paper to obtain the solid aspirin product. The liquid filtrate contains acetic acid and can be discarded.
- Purification: The solid aspirin product is rinsed with cold water to remove any impurities and then dried using a watch glass or by placing in an oven or desiccator.
- Yield calculation: The weight of the aspirin product is measured and the percent yield is calculated.
- Recrystallization (optional): The aspirin product can be further purified by recrystallization in ethanol.
It is important to note that the synthesis of aspirin from phenols requires the use of hazardous chemicals and should only be performed under appropriate laboratory conditions with proper safety precautions.
Nomenclature of Phenols Aspirin synthesis
In the context of the synthesis of aspirin from phenols, the nomenclature of the phenol used as a starting material follows the standard IUPAC rules for naming organic compounds.
The IUPAC name of a phenol consists of the prefix “phenol” followed by the name of the substituent attached to the phenol ring. For example, if the substituent is a methyl group (-CH3), the compound would be named “methylphenol.”
In the case of the synthesis of aspirin, the phenol used is typically just referred to as “phenol” or “hydroxybenzene.”
It is important to note that the naming of organic compounds can become more complex depending on the number and position of substituents on the phenol ring, and the IUPAC nomenclature system provides a standardized method for naming these compounds.
Case Study on Phenols Aspirin synthesis
Here is an example case study on the synthesis of aspirin from phenols:
Case Study: Synthesis of Aspirin from Phenol
A group of undergraduate students in an organic chemistry laboratory course were tasked with synthesizing aspirin from phenol. The students were provided with the necessary reagents and equipment, and given the following procedure to follow:
- In a 100 mL beaker, mix 3 g of phenol with 4 mL of acetic anhydride and add 0.5 mL of sulfuric acid catalyst. Stir the mixture with a glass rod.
- Heat the beaker on a hot plate at 50-55°C for 10 minutes, stirring continuously.
- Remove the beaker from the heat and allow it to cool to room temperature.
- Slowly add 20 mL of cold water to the mixture while stirring continuously.
- Add a small amount of sodium bicarbonate to adjust the pH to around 7-8.
- Filter the mixture through filter paper and collect the solid aspirin product.
- Rinse the aspirin product with cold water to remove any impurities and dry using a watch glass or by placing in an oven or desiccator.
The students followed the procedure carefully and obtained a white solid product with a yield of 2.5 g, which corresponded to a percent yield of approximately 83%. They also obtained a melting point of 136-138°C for the product, which was consistent with the expected melting point range for aspirin.
The students then performed a recrystallization of the product in ethanol to further purify the aspirin, and obtained a final product with a melting point of 137-139°C and a yield of 2.2 g.
The students concluded that the synthesis of aspirin from phenol was successful, and they were able to obtain a reasonably high yield of pure aspirin using the provided procedure. They also learned about the importance of safety measures and proper waste disposal when working with hazardous chemicals in a laboratory setting.
White paper on Phenols Aspirin synthesis
Here is a white paper on the synthesis of aspirin from phenols:
Introduction
Aspirin, also known as acetylsalicylic acid, is a commonly used medication for pain relief and fever reduction. It is synthesized from salicylic acid, which is found naturally in plants such as willow trees. The synthesis of aspirin from salicylic acid involves the reaction of salicylic acid with acetic anhydride in the presence of a catalyst, typically sulfuric acid. However, salicylic acid is a phenolic compound and can also be synthesized into aspirin via a direct esterification reaction with acetic anhydride. In this white paper, we will explore the synthesis of aspirin from phenols, specifically using phenol as a starting material.
Synthesis of Aspirin from Phenols
The synthesis of aspirin from phenols involves the reaction of phenol with acetic anhydride in the presence of a catalyst, typically sulfuric acid. The reaction proceeds via an esterification mechanism, where the hydroxyl group of phenol reacts with the acetic anhydride to form acetylsalicylic acid and acetic acid. The sulfuric acid catalyst helps to facilitate the reaction by protonating the hydroxyl group of phenol, making it more reactive towards the acetic anhydride.
The reaction can be summarized as follows:
Phenol + Acetic Anhydride → Acetylsalicylic Acid + Acetic Acid
The reaction is exothermic and typically carried out at a temperature of 50-55°C. After the reaction is complete, the mixture is allowed to cool and then hydrolyzed with cold water to remove excess acetic anhydride and acetic acid. Sodium bicarbonate is added to adjust the pH of the mixture to around 7-8, and the solid aspirin product is filtered and purified.
Purification of Aspirin
The aspirin product obtained from the synthesis reaction is typically impure and requires further purification. The product is first rinsed with cold water to remove any impurities, and then dried using a watch glass or by placing in an oven or desiccator. If necessary, the product can be further purified through recrystallization in a suitable solvent such as ethanol.
Safety Considerations
The synthesis of aspirin from phenols involves the use of hazardous chemicals, including acetic anhydride and sulfuric acid. It is important to follow proper safety precautions when working with these chemicals, including wearing appropriate personal protective equipment such as gloves and safety glasses, working in a well-ventilated area, and disposing of waste materials properly.
Conclusion
The synthesis of aspirin from phenols is a straightforward and efficient method for producing aspirin from a phenolic starting material. The reaction can be carried out using common laboratory equipment and reagents, and the resulting aspirin product can be purified through simple methods such as filtration and recrystallization. However, it is important to exercise caution and follow proper safety procedures when working with hazardous chemicals in a laboratory setting.
