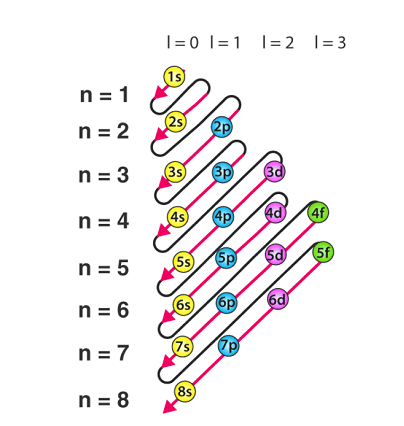
The Aufbau principle, also known as the Aufbau rule, is a fundamental concept in chemistry that explains the order in which electrons fill atomic orbitals in an atom. It is based on the idea that electrons occupy the lowest available energy level or orbital first, before filling higher levels or orbitals.
According to the Aufbau principle, the order of filling of atomic orbitals is determined by their energy levels. The energy level of an orbital is primarily determined by its distance from the nucleus and the amount of shielding provided by the other electrons in the atom. Orbitals with lower energy levels are closer to the nucleus and are shielded less, while orbitals with higher energy levels are further from the nucleus and are shielded more.
The order of filling of atomic orbitals is as follows:
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p.
In other words, the lowest energy level orbital, the 1s orbital, is filled first, followed by the 2s and then the 2p orbitals, and so on. This principle helps to explain the electron configuration of elements in the periodic table and their chemical properties.
What is Required Aufbau Principle
The Required Aufbau Principle is a modified version of the Aufbau principle that takes into account the effects of electron-electron repulsion on the filling of atomic orbitals. While the Aufbau principle states that electrons fill orbitals in order of increasing energy, the Required Aufbau Principle takes into account the fact that some electron configurations are more stable than others due to electron repulsion.
According to the Required Aufbau Principle, when filling atomic orbitals, electrons first occupy the orbitals with the lowest energy, but when multiple orbitals have the same energy, the orbitals are filled with electrons in such a way as to maximize the number of unpaired electrons. This is because electrons in paired orbitals repel each other, which makes the configuration less stable.
For example, the electron configuration of chromium (Cr) is [Ar] 3d^5 4s^1 instead of [Ar] 3d^4 4s^2 as would be predicted by the simple Aufbau principle. This is because the 3d orbitals are split into two sublevels, one of which is slightly lower in energy than the other. By filling the lower energy 3d sublevel with five electrons, chromium achieves a half-filled sublevel with maximum unpaired electrons, which is a more stable configuration than having four paired electrons in the 3d sublevel.
The Required Aufbau Principle helps to explain why certain electron configurations are more stable than others, and why some elements have exceptions to the predicted electron configuration based on the simple Aufbau principle.
When is Required Aufbau Principle
The Required Aufbau Principle is a concept in chemistry that is always applicable when considering the electron configuration of atoms. It is a modification of the Aufbau principle that takes into account the effects of electron-electron repulsion on the filling of atomic orbitals. The Required Aufbau Principle helps to explain why certain electron configurations are more stable than others, and why some elements have exceptions to the predicted electron configuration based on the simple Aufbau principle. Therefore, the Required Aufbau Principle is relevant in all contexts where electron configurations are being considered, from basic chemistry to advanced applications such as materials science, solid-state physics, and spectroscopy.
Production of Aufbau Principle
The Aufbau principle is a theoretical concept in chemistry that describes the order in which electrons fill atomic orbitals. It is not produced or manufactured in a physical sense, but rather it is a fundamental principle that has been derived from experimental observations and theoretical calculations. The Aufbau principle was first proposed by Arnold Sommerfeld in 1922 and has since been refined and expanded upon by numerous scientists in the field of chemistry.
The principle itself is based on the idea that electrons occupy the lowest energy orbitals first and then fill higher energy orbitals as needed. This results in a sequence of electron configurations that can be predicted based on the periodic table and the number of electrons in an atom. The principle has been confirmed by numerous experimental observations and is widely accepted as a fundamental concept in chemistry.
In summary, the Aufbau principle is not produced or manufactured, but rather it is a fundamental principle that has been derived from experimental observations and theoretical calculations. It is an essential concept in chemistry that helps to explain the electronic structure of atoms and molecules, and has wide applications in various fields of chemistry.
Where is Required Aufbau Principle
The Required Aufbau Principle is a concept in chemistry that is not physically located in a particular place. It is a modification of the Aufbau principle, which is a fundamental concept in chemistry that explains the order in which electrons fill atomic orbitals in an atom. The Required Aufbau Principle takes into account the effects of electron-electron repulsion on the filling of atomic orbitals and helps to explain why certain electron configurations are more stable than others.
The Required Aufbau Principle is a theoretical concept that is applied to the understanding of electron configurations of atoms and molecules. It is used in various fields of chemistry, including materials science, solid-state physics, and spectroscopy, to explain the properties and behavior of different chemical systems. So, the Required Aufbau Principle is not a physical object or a place but a theoretical concept that is widely used in chemistry.
How is Required Aufbau Principle
The Required Aufbau Principle is a modification of the Aufbau principle, which is a fundamental concept in chemistry that explains the order in which electrons fill atomic orbitals in an atom. The Required Aufbau Principle takes into account the effects of electron-electron repulsion on the filling of atomic orbitals, which can have a significant impact on the stability of an electron configuration.
The Required Aufbau Principle is based on the idea that when filling atomic orbitals, electrons first occupy the orbitals with the lowest energy, but when multiple orbitals have the same energy, the orbitals are filled with electrons in such a way as to maximize the number of unpaired electrons. This is because electrons in paired orbitals repel each other, which makes the configuration less stable.
For example, the electron configuration of chromium (Cr) is [Ar] 3d^5 4s^1 instead of [Ar] 3d^4 4s^2 as would be predicted by the simple Aufbau principle. This is because the 3d orbitals are split into two sublevels, one of which is slightly lower in energy than the other. By filling the lower energy 3d sublevel with five electrons, chromium achieves a half-filled sublevel with maximum unpaired electrons, which is a more stable configuration than having four paired electrons in the 3d sublevel.
The Required Aufbau Principle is important because it helps to explain why certain electron configurations are more stable than others, and why some elements have exceptions to the predicted electron configuration based on the simple Aufbau principle. It is a theoretical concept that is applied to the understanding of electron configurations of atoms and molecules in various fields of chemistry, including materials science, solid-state physics, and spectroscopy.
Case Study on Aufbau Principle
The Aufbau principle is a fundamental concept in chemistry that explains the order in which electrons fill atomic orbitals in an atom. It is an important principle that has wide applications in various fields of chemistry, including materials science, solid-state physics, and spectroscopy. One interesting case study that illustrates the application of the Aufbau principle is the electron configuration of copper (Cu).
Copper is a transition metal that has an atomic number of 29. According to the Aufbau principle, the electron configuration of copper should be [Ar] 3d^9 4s^2. However, experimental data shows that the actual electron configuration of copper is [Ar] 3d^10 4s^1. This means that one electron from the 4s orbital has moved to the 3d orbital, resulting in a full 3d subshell.
This deviation from the predicted electron configuration based on the Aufbau principle can be explained by the fact that the 3d and 4s orbitals have very similar energies. This means that when filling the electron configuration of copper, the electrons in the 4s orbital can easily move to the 3d orbital, resulting in a more stable configuration. This is because the 3d orbital has more unpaired electrons, which results in stronger electron-electron repulsion and a less stable configuration.
The deviation from the predicted electron configuration based on the Aufbau principle is not unique to copper. Many other transition metals, such as chromium and manganese, also have exceptions to the predicted electron configuration due to the similar energies of the 3d and 4s orbitals. These exceptions are important to understand because they have a significant impact on the properties and behavior of these elements and their compounds.
In summary, the case study of copper illustrates the application of the Aufbau principle in understanding electron configurations of atoms and how deviations from the predicted configurations can arise due to similar energies of atomic orbitals. The Aufbau principle is a fundamental concept that is widely used in chemistry to explain the properties and behavior of different chemical systems.
White paper on Aufbau Principle
Introduction:
The Aufbau principle is a fundamental concept in chemistry that explains the order in which electrons fill atomic orbitals in an atom. It is a principle that is used to understand the electronic structure of atoms and how this structure affects the properties and behavior of different chemical systems. In this white paper, we will provide an overview of the Aufbau principle, its historical background, and its applications in various fields of chemistry.
Background:
The Aufbau principle, also known as the building-up principle, was first proposed by the German physicist Arnold Sommerfeld in 1922. The principle is based on the idea that when filling atomic orbitals, electrons first occupy the orbitals with the lowest energy and then fill higher energy orbitals as needed. This results in a sequence of electron configurations that can be predicted based on the periodic table and the number of electrons in an atom.
The simple Aufbau principle predicts the electron configuration of atoms by filling the orbitals in order of increasing energy. For example, the electron configuration of oxygen (O) is 1s^2 2s^2 2p^4, where the 1s orbital is filled first, followed by the 2s and then the 2p orbitals. This principle works well for most atoms, but there are exceptions that require a more refined version of the principle.
The Required Aufbau Principle:
The Required Aufbau Principle is a modification of the simple Aufbau principle that takes into account the effects of electron-electron repulsion on the filling of atomic orbitals. This principle is based on the idea that when multiple orbitals have the same energy, the orbitals are filled with electrons in such a way as to maximize the number of unpaired electrons. This is because electrons in paired orbitals repel each other, which makes the configuration less stable.
The Required Aufbau Principle helps to explain why certain electron configurations are more stable than others and why some elements have exceptions to the predicted electron configuration based on the simple Aufbau principle. For example, the electron configuration of chromium (Cr) is [Ar] 3d^5 4s^1 instead of [Ar] 3d^4 4s^2 as would be predicted by the simple Aufbau principle. This is because the 3d orbitals are split into two sublevels, one of which is slightly lower in energy than the other. By filling the lower energy 3d sublevel with five electrons, chromium achieves a half-filled sublevel with maximum unpaired electrons, which is a more stable configuration than having four paired electrons in the 3d sublevel.
Applications:
The Aufbau principle and the Required Aufbau Principle are important principles in chemistry that have wide applications in various fields. For example, the understanding of the electron configuration of atoms is essential in the design of new materials with specific properties. Materials scientists use the principles of chemistry to develop new materials with improved properties, such as better conductivity or increased strength.
The principles of the Aufbau principle are also important in spectroscopy, which is the study of the interaction of electromagnetic radiation with matter. Spectroscopy is used to identify and quantify the composition of matter, including atoms and molecules. The electronic structure of atoms and molecules determines their interaction with electromagnetic radiation, and the principles of the Aufbau principle are essential in understanding these interactions.
Conclusion:
In conclusion, the Aufbau principle is a fundamental concept in chemistry that explains the order in which electrons fill atomic orbitals in an atom. This principle is based on the idea that electrons first occupy the orbitals with the lowest energy and then fill higher energy orbitals as needed. The Required Aufbau Principle is a modification of the simple Aufbau principle that takes into account the effects of electron-electron repulsion on the filling of atomic orbitals. These principles are essential in understanding the electronic structure of atoms and molecules and have wide applications in various fields of chemistry. By understanding these principles, scientists can design new materials with specific properties and use spectroscopy to identify and quantify the composition of matter.
