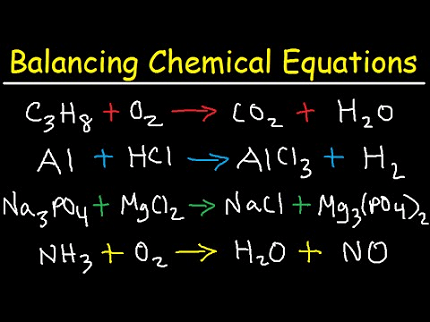
A balanced chemical equation is a representation of a chemical reaction using chemical formulas and symbols. It shows the reactants on the left side of the equation and the products on the right side of the equation.
The key feature of a balanced chemical equation is that the number of atoms of each element is the same on both sides of the equation. This means that the law of conservation of mass is obeyed, which states that matter cannot be created or destroyed in a chemical reaction, only rearranged.
Balancing a chemical equation involves adjusting the coefficients in front of the chemical formulas so that the number of atoms of each element is the same on both sides of the equation. This is done by using mathematical principles and chemical knowledge to determine the correct coefficients.
For example, the balanced chemical equation for the reaction between hydrogen gas (H2) and oxygen gas (O2) to form water (H2O) is:
2 H2 + O2 → 2 H2O
In this equation, there are two atoms of hydrogen and two atoms of oxygen on both sides of the equation, which means that the equation is balanced.
What is Required Balanced chemical equations
A balanced chemical equation is required in order to accurately represent a chemical reaction. It ensures that the law of conservation of mass is obeyed, which states that the total mass of the reactants must be equal to the total mass of the products in a chemical reaction.
A balanced chemical equation provides information about the relative amounts of reactants and products involved in the reaction. It also helps to predict the outcome of the reaction, as well as the amount of energy that is released or absorbed during the reaction.
In addition, a balanced chemical equation is essential for stoichiometry calculations, which involve determining the amount of reactants or products involved in a chemical reaction. Without a balanced equation, stoichiometry calculations cannot be accurately performed.
Therefore, a balanced chemical equation is a fundamental tool in chemistry, and it is essential for understanding and predicting chemical reactions.
Who is Required Balanced chemical equations
Balanced chemical equations are required by anyone who wants to accurately represent, understand, and predict chemical reactions. This includes:
- Chemists: Chemists use balanced chemical equations to describe the chemical reactions they are studying in the lab or in theoretical models. They use these equations to determine the amounts of reactants and products, predict reaction outcomes, and calculate reaction rates.
- Students: Students studying chemistry at any level, from high school to university, are required to learn how to balance chemical equations. This is a fundamental skill that is necessary for understanding the basic principles of chemistry.
- Engineers: Engineers who work in fields such as materials science, chemical engineering, and environmental engineering use balanced chemical equations to design and optimize industrial processes. They use these equations to determine the optimal reaction conditions, the amount of reactants needed, and the expected yields of products.
- Researchers: Researchers in fields such as pharmaceuticals, biotechnology, and materials science use balanced chemical equations to design new compounds and materials. They use these equations to predict the properties of new materials and to optimize synthesis routes to achieve the desired properties.
In summary, balanced chemical equations are required by anyone who wants to accurately describe and understand chemical reactions, from chemists and students to engineers and researchers.
When is Required Balanced chemical equations
A balanced chemical equation is required in any situation where there is a chemical reaction taking place. Some specific situations where a balanced chemical equation is necessary include:
- Chemical reactions in the laboratory: Chemists need balanced chemical equations to accurately represent the reactions they are studying in the lab. This allows them to determine the amounts of reactants and products involved in the reaction, predict reaction outcomes, and calculate reaction rates.
- Industrial processes: Engineers in fields such as chemical engineering, materials science, and environmental engineering use balanced chemical equations to design and optimize industrial processes. This helps them to determine the optimal reaction conditions, the amount of reactants needed, and the expected yields of products.
- Pharmaceutical research: Researchers in the pharmaceutical industry use balanced chemical equations to design and synthesize new compounds for use as drugs. This allows them to predict the properties of the compounds and optimize their synthesis routes to achieve the desired properties.
- Environmental analysis: Environmental scientists and engineers use balanced chemical equations to understand and predict the fate and transport of pollutants in the environment. This helps them to design effective remediation strategies to reduce the impact of pollutants on ecosystems and human health.
In summary, a balanced chemical equation is required in any situation where there is a chemical reaction taking place, including in the laboratory, in industrial processes, in pharmaceutical research, and in environmental analysis.
Where is Required Balanced chemical equations
A balanced chemical equation is required wherever there is a chemical reaction taking place. This includes a variety of different settings, such as:
- Laboratory: Chemists use balanced chemical equations in the laboratory to represent the reactions they are studying. This allows them to understand the reaction mechanism, predict the products that will be formed, and calculate reaction rates.
- Industrial processes: Engineers in industries such as chemical manufacturing, materials science, and biotechnology use balanced chemical equations to design and optimize industrial processes. This helps them to determine the optimal reaction conditions, the amount of reactants needed, and the expected yields of products.
- Environmental analysis: Environmental scientists and engineers use balanced chemical equations to understand and predict the fate and transport of pollutants in the environment. This allows them to design effective remediation strategies to reduce the impact of pollutants on ecosystems and human health.
- Academic settings: Students of chemistry at all levels, from high school to university, are required to learn how to balance chemical equations. This is a fundamental skill that is necessary for understanding the basic principles of chemistry.
In summary, a balanced chemical equation is required wherever there is a chemical reaction taking place, including in the laboratory, in industrial processes, in environmental analysis, and in academic settings.
How is Required Balanced chemical equations
Balancing a chemical equation involves adjusting the coefficients of the reactants and products so that the law of conservation of mass is obeyed. This means that the total mass of the reactants must be equal to the total mass of the products in a chemical reaction.
The following steps can be used to balance a chemical equation:
- Write the chemical equation: Start by writing the chemical equation for the reaction, including the reactants and products. Be sure to include the correct chemical formulas for each substance.
- Count the atoms: Count the number of atoms of each element on both sides of the equation. This will help you to determine which coefficients need to be adjusted.
- Start with the most complex molecule: Choose one of the most complex molecules in the equation and balance it first. This will often involve adjusting the coefficients of multiple atoms at once.
- Balance the other molecules: Once the most complex molecule has been balanced, move on to the other molecules in the equation and balance them one at a time. This may involve adjusting the coefficients of one or more atoms in each molecule.
- Check the equation: Once you have balanced all of the molecules in the equation, check to make sure that the number of atoms of each element is the same on both sides of the equation.
- Use whole numbers: If any coefficients are fractions, multiply the entire equation by the smallest whole number that will eliminate the fractions.
- Optional: Include state symbols: If desired, include state symbols (s, l, g, or aq) to indicate the physical state of each substance in the equation.
In summary, balancing a chemical equation involves adjusting the coefficients of the reactants and products so that the law of conservation of mass is obeyed. This is done by counting the atoms of each element, starting with the most complex molecule, and balancing the other molecules one at a time.
Case Study on Balanced chemical equations
Case study: Balancing a chemical equation in a laboratory setting
A laboratory chemist is studying a chemical reaction between copper sulfate (CuSO4) and iron (Fe). The reaction is as follows:
CuSO4 + Fe → Cu + FeSO4
The chemist notices that the equation is not balanced, as there are different numbers of atoms of each element on both sides of the equation. They need to balance the equation to accurately represent the reaction and determine the amounts of reactants and products involved.
The chemist follows the following steps to balance the chemical equation:
- Write the chemical equation: The chemist writes the chemical equation for the reaction as CuSO4 + Fe → Cu + FeSO4.
- Count the atoms: They count the number of atoms of each element on both sides of the equation. There are one copper (Cu) atom, one iron (Fe) atom, one sulfur (S) atom, and four oxygen (O) atoms on the left side, and one copper (Cu) atom, one iron (Fe) atom, one sulfur (S) atom, and four oxygen (O) atoms on the right side.
- Start with the most complex molecule: The chemist decides to start by balancing the sulfate (SO4) ion. They add a coefficient of 1 to CuSO4, so the number of sulfur atoms on the left side becomes one.
- Balance the other molecules: The chemist then balances the iron (Fe) atoms by adding a coefficient of 1 to Fe on the right side.
- Check the equation: They check to make sure that the number of atoms of each element is the same on both sides of the equation. They notice that the equation is now balanced.
- Use whole numbers: Since all coefficients are already whole numbers, there is no need to multiply the equation by any factor to eliminate fractional coefficients.
- Optional: Include state symbols: The chemist decides to include state symbols to indicate that CuSO4 is a solid (s) and FeSO4 is an aqueous solution (aq).
The balanced chemical equation for the reaction is:
CuSO4 (s) + Fe (s) → Cu (s) + FeSO4 (aq)
By balancing the chemical equation, the chemist is now able to accurately represent the reaction and determine the amounts of reactants and products involved. This allows them to study the reaction mechanism, predict the products that will be formed, and calculate reaction rates, among other things.
White paper on Balanced chemical equations
Introduction
Balanced chemical equations are an essential tool in chemistry, allowing us to accurately represent chemical reactions and understand the underlying processes. In this white paper, we will discuss the importance of balanced chemical equations, their role in chemical calculations, and the process of balancing a chemical equation.
Importance of Balanced Chemical Equations
Balanced chemical equations are important because they represent a fundamental principle in chemistry: the law of conservation of mass. This law states that the total mass of the reactants in a chemical reaction must be equal to the total mass of the products. By balancing a chemical equation, we ensure that this law is obeyed, and we can accurately represent the stoichiometry of the reaction.
Balanced chemical equations are also important in predicting the outcome of a reaction. By knowing the amounts of reactants and products involved, we can predict which reactant will be limiting, and how much of each product will be formed.
In addition, balanced chemical equations are used in a wide range of chemical calculations, including determining reaction yields, calculating the concentration of solutions, and predicting the amount of heat released or absorbed during a reaction.
The Process of Balancing a Chemical Equation
Balancing a chemical equation involves adjusting the coefficients of the reactants and products to ensure that the number of atoms of each element is the same on both sides of the equation. This process can be broken down into several steps:
- Write the chemical equation: Start by writing the chemical equation for the reaction, including the correct chemical formulas for each substance.
- Count the atoms: Count the number of atoms of each element on both sides of the equation. This will help you to determine which coefficients need to be adjusted.
- Start with the most complex molecule: Choose one of the most complex molecules in the equation and balance it first. This will often involve adjusting the coefficients of multiple atoms at once.
- Balance the other molecules: Once the most complex molecule has been balanced, move on to the other molecules in the equation and balance them one at a time. This may involve adjusting the coefficients of one or more atoms in each molecule.
- Check the equation: Once you have balanced all of the molecules in the equation, check to make sure that the number of atoms of each element is the same on both sides of the equation.
- Use whole numbers: If any coefficients are fractions, multiply the entire equation by the smallest whole number that will eliminate the fractions.
- Optional: Include state symbols: If desired, include state symbols (s, l, g, or aq) to indicate the physical state of each substance in the equation.
Conclusion
Balanced chemical equations are an essential tool in chemistry, allowing us to accurately represent chemical reactions and understand the underlying processes. By balancing a chemical equation, we ensure that the law of conservation of mass is obeyed, and we can accurately predict the outcome of a reaction and perform a wide range of chemical calculations. The process of balancing a chemical equation involves adjusting the coefficients of the reactants and products to ensure that the number of atoms of each element is the same on both sides of the equation.
