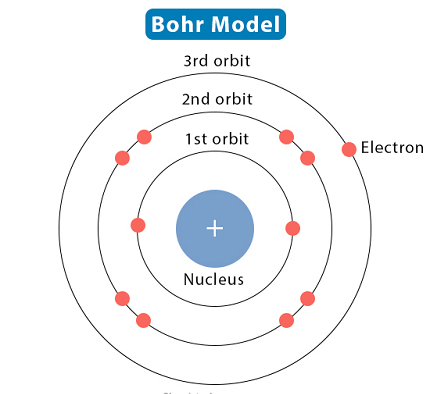
The Bohr model is a simple model of the atom proposed by Danish physicist Niels Bohr in 1913. It was an early attempt to explain the behavior of electrons in an atom and the spectrum of light emitted by atoms.
The Bohr model posits that electrons move in circular orbits around the nucleus of an atom, similar to the way that planets orbit around the sun. Bohr also proposed that electrons could only occupy certain, discrete energy levels, and that when an electron moves from a higher energy level to a lower one, it emits a photon of light.
The Bohr model was significant because it provided a way to explain the line spectra of different elements, which was a major puzzle for physicists at the time. However, the Bohr model was later found to be an oversimplification, and was replaced by more sophisticated models, such as the quantum mechanical model of the atom.
What is Required Bohr model Atomic Structure
The Bohr model of atomic structure requires that the atom has a central nucleus consisting of protons and neutrons, with electrons orbiting around the nucleus in circular orbits.
According to the Bohr model, each electron has a specific, quantized energy level, which is determined by the distance of the electron from the nucleus. Electrons in the outermost energy level are called valence electrons, and they determine the chemical properties of the element.
The Bohr model also requires that the number of electrons in an atom is equal to the number of protons in the nucleus, giving the atom a net charge of zero. The number of protons in the nucleus is known as the atomic number, and it determines the identity of the element.
While the Bohr model was useful for explaining some of the behavior of atoms, it was later found to be an oversimplification. The quantum mechanical model of atomic structure, which is more sophisticated and accurate, was developed to replace it.
Who is Required Bohr model Atomic Structure
The Bohr model of atomic structure was proposed by Danish physicist Niels Bohr in 1913. Bohr was working at the time in Ernest Rutherford’s laboratory at the University of Manchester, where he was studying the behavior of electrons in atoms.
Bohr’s work on the Bohr model of atomic structure was significant because it provided a way to explain the line spectra of different elements, which was a major puzzle for physicists at the time. The model also helped to lay the foundation for the development of quantum mechanics, which revolutionized our understanding of the behavior of atoms and subatomic particles.
For his work on atomic structure, Bohr was awarded the Nobel Prize in Physics in 1922. He went on to make many other contributions to physics and became a leading figure in the development of quantum mechanics.
When is Required Bohr model Atomic Structure
The Bohr model of atomic structure was proposed by Niels Bohr in 1913. At that time, there was a great deal of interest in understanding the behavior of electrons in atoms, particularly the way that atoms emit light in discrete spectral lines.
Bohr’s model was one of the first attempts to explain the behavior of electrons in an atom in a way that was consistent with experimental observations. The model helped to explain the line spectra of different elements, which was a major puzzle for physicists at the time.
The Bohr model of atomic structure was a significant step forward in our understanding of atoms and their behavior. However, it was later found to be an oversimplification, and was replaced by more sophisticated models, such as the quantum mechanical model of the atom. Nonetheless, the Bohr model remains an important historical landmark in the development of atomic theory.
Where is Required Bohr model Atomic Structure
The Bohr model of atomic structure was proposed by Niels Bohr while he was working at the University of Copenhagen in Denmark. Bohr was a Danish physicist who made many important contributions to the field of physics, including the development of the Bohr model of atomic structure.
Bohr’s work on atomic structure was influenced by the research of other physicists, including Ernest Rutherford, who had discovered the atomic nucleus and proposed a model of the atom that consisted of a central nucleus surrounded by orbiting electrons.
Bohr’s model built on Rutherford’s work by proposing that electrons could only occupy certain, discrete energy levels, and that when an electron moves from a higher energy level to a lower one, it emits a photon of light. This helped to explain the line spectra of different elements, which was a major puzzle for physicists at the time.
The Bohr model of atomic structure was developed in Denmark, but its impact was felt throughout the world of physics, and it helped to lay the foundation for the development of quantum mechanics.
How is Required Bohr model Atomic Structure
The Bohr model of atomic structure describes the structure of an atom in terms of a central nucleus, consisting of protons and neutrons, and electrons that orbit around the nucleus in circular orbits, much like planets orbiting around the sun.
The model also posits that electrons can only occupy certain, discrete energy levels, which are determined by the distance of the electron from the nucleus. Electrons in the outermost energy level, called the valence shell, determine the chemical properties of the element.
Bohr’s model proposed that when an electron moves from a higher energy level to a lower one, it emits a photon of light. This helped to explain the line spectra of different elements, which was a major puzzle for physicists at the time.
The Bohr model also requires that the number of electrons in an atom is equal to the number of protons in the nucleus, giving the atom a net charge of zero. The number of protons in the nucleus is known as the atomic number, and it determines the identity of the element.
While the Bohr model was a significant step forward in our understanding of atomic structure, it was later found to be an oversimplification. More sophisticated models, such as the quantum mechanical model of the atom, were developed to replace it.
Case Study on Bohr model Atomic Structure
One of the most famous applications of the Bohr model of atomic structure is in explaining the line spectra of different elements. In the early 20th century, physicists were studying the light emitted by various elements and noticed that each element produced a unique set of discrete spectral lines.
Bohr’s model of atomic structure proposed that electrons orbit around the nucleus in circular orbits, much like planets orbiting around the sun. He also proposed that electrons could only occupy certain, discrete energy levels, and that when an electron moved from a higher energy level to a lower one, it emitted a photon of light.
Using this model, Bohr was able to explain the line spectra of different elements. The lines in the spectra corresponded to the different energy transitions that electrons could undergo as they moved between different energy levels. The wavelengths of the emitted light could be calculated using the formula:
λ = hc/ΔE
where λ is the wavelength of the emitted light, h is Planck’s constant, c is the speed of light, and ΔE is the difference in energy between the initial and final energy levels of the electron.
This formula was able to accurately predict the wavelengths of the spectral lines for a wide range of elements, providing strong support for the Bohr model of atomic structure.
While the Bohr model was later found to be an oversimplification, it remains an important historical landmark in the development of atomic theory. It helped to lay the foundation for the development of quantum mechanics, which has provided us with a much more sophisticated understanding of the behavior of atoms and subatomic particles.
White paper on Bohr model Atomic Structure
Introduction
The Bohr model of atomic structure was proposed by Danish physicist Niels Bohr in 1913. At the time, there was a great deal of interest in understanding the behavior of electrons in atoms, particularly the way that atoms emit light in discrete spectral lines. Bohr’s model was one of the first attempts to explain the behavior of electrons in an atom in a way that was consistent with experimental observations. This white paper will provide an in-depth analysis of the Bohr model of atomic structure, including its history, key concepts, and limitations.
History of the Bohr Model
The development of the Bohr model of atomic structure was influenced by the research of other physicists, including Ernest Rutherford, who had discovered the atomic nucleus and proposed a model of the atom that consisted of a central nucleus surrounded by orbiting electrons. Bohr’s work built on Rutherford’s model by proposing that electrons could only occupy certain, discrete energy levels, and that when an electron moves from a higher energy level to a lower one, it emits a photon of light.
Bohr’s model helped to explain the line spectra of different elements, which was a major puzzle for physicists at the time. The lines in the spectra corresponded to the different energy transitions that electrons could undergo as they moved between different energy levels. Bohr’s model was able to accurately predict the wavelengths of the spectral lines for a wide range of elements, providing strong support for the model.
Key Concepts of the Bohr Model
The Bohr model of atomic structure describes the structure of an atom in terms of a central nucleus, consisting of protons and neutrons, and electrons that orbit around the nucleus in circular orbits, much like planets orbiting around the sun. The model also posits that electrons can only occupy certain, discrete energy levels, which are determined by the distance of the electron from the nucleus. Electrons in the outermost energy level, called the valence shell, determine the chemical properties of the element.
Bohr’s model proposed that when an electron moves from a higher energy level to a lower one, it emits a photon of light. This helped to explain the line spectra of different elements, which was a major puzzle for physicists at the time. The Bohr model also requires that the number of electrons in an atom is equal to the number of protons in the nucleus, giving the atom a net charge of zero. The number of protons in the nucleus is known as the atomic number, and it determines the identity of the element.
Limitations of the Bohr Model
While the Bohr model was a significant step forward in our understanding of atomic structure, it was later found to be an oversimplification. One of the main limitations of the model was that it did not account for the wave-like behavior of electrons. This led to the development of more sophisticated models, such as the quantum mechanical model of the atom, which took into account the wave-particle duality of electrons.
Another limitation of the Bohr model was that it did not accurately predict the behavior of atoms with more than one electron. The interactions between electrons in these atoms are complex and cannot be explained by the simple circular orbits of the Bohr model. Nonetheless, the Bohr model remains an important historical landmark in the development of atomic theory.
Conclusion
The Bohr model of atomic structure, proposed by Niels Bohr in 1913, was a significant step forward in our understanding of atoms and their behavior. The model helped to explain the line spectra of different elements, which was a major puzzle for physicists at the time. However, the model was later found to be an oversimplification and was replaced by more sophisticated models, such as the quantum mechanical model of the atom. Nonetheless, the Bohr model remains an important historical landmark in the development of atomic theory and continues to be studied and taught in physics and chemistry. The key concepts of the model, including the discrete energy levels of electrons and the valence shell, remain relevant in our understanding of atoms and their chemical properties.
