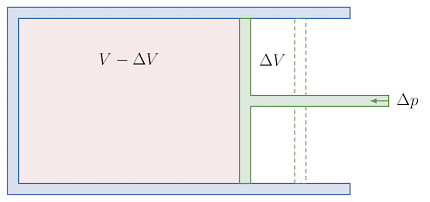
The bulk modulus of a gas is a measure of the gas’s resistance to compression. It is defined as the ratio of the change in pressure to the fractional change in volume, under conditions of constant temperature.
For an ideal gas, the bulk modulus can be calculated using the ideal gas law, which relates the pressure, volume, and temperature of a gas. The bulk modulus of an ideal gas is given by:
K = p
where K is the bulk modulus, and p is the pressure of the gas.
Note that the bulk modulus of a gas is much lower than that of a solid or a liquid, because gases are much more compressible than solids or liquids.
What is Required Bulk modulus of gases
The required bulk modulus of a gas depends on the specific application or situation in which the gas is being used.
For example, in some industrial processes, gases may need to be compressed or expanded rapidly, so a higher bulk modulus may be required to ensure that the gas responds quickly to changes in pressure or volume. In other cases, such as in gas storage tanks, a lower bulk modulus may be acceptable because the gas is stored at a relatively constant pressure and temperature.
In general, the bulk modulus of a gas is not as critical a factor as some of its other properties, such as its density, specific heat, and thermal conductivity, which can have a significant impact on the performance of a system or process in which the gas is used.
When is Required Bulk modulus of gases
The required bulk modulus of gases can be important in various engineering applications where gases are compressed, expanded, or used as a medium for transmitting pressure.
Some examples of applications where the bulk modulus of gases may be a significant factor include:
- Gas compressors: In gas compression systems, the bulk modulus of the gas can affect the efficiency of the compressor and the rate at which gas can be compressed.
- Pneumatic systems: In pneumatic systems, the bulk modulus of the gas can affect the response time of the system and its ability to maintain a constant pressure.
- Gas storage tanks: In gas storage tanks, the bulk modulus of the gas can affect the rate at which gas is stored and released, as well as the pressure fluctuations that occur during storage and usage.
- Gas pipelines: In gas pipelines, the bulk modulus of the gas can affect the transmission of pressure and the ability of the pipeline to maintain a constant flow rate.
- Shock absorbers: In shock absorbers, the bulk modulus of the gas can affect the system’s ability to absorb and dissipate energy during impacts.
In general, the required bulk modulus of gases will depend on the specific application and the performance requirements of the system or process in which the gas is used.
Where is Required Bulk modulus of gases
The required bulk modulus of gases can be important in various engineering and scientific fields where gases are used. Some of the areas where the bulk modulus of gases is important include:
- Mechanical engineering: In mechanical engineering, the bulk modulus of gases is important in the design and operation of gas compressors, pneumatic systems, shock absorbers, and other devices that use gases.
- Chemical engineering: In chemical engineering, the bulk modulus of gases is important in the design and operation of gas storage tanks, gas pipelines, and other systems that transport or store gases.
- Physics: In physics, the bulk modulus of gases is important in the study of fluid dynamics and the behavior of gases under different pressure and temperature conditions.
- Materials science: In materials science, the bulk modulus of gases is important in the design and synthesis of porous materials, such as zeolites, which can be used for gas storage and separation.
In general, the required bulk modulus of gases will depend on the specific application and the performance requirements of the system or process in which the gas is used.
How is Required Bulk modulus of gases
The required bulk modulus of gases is determined by the specific application or system in which the gas is being used.
To determine the required bulk modulus of a gas for a particular application, several factors must be considered, such as the pressure and temperature ranges that the gas will be exposed to, the rate of compression or expansion required, the response time of the system, and the stability and accuracy of the pressure or flow control.
Once these factors are known, the bulk modulus can be calculated or estimated using the ideal gas law or other equations of state. In some cases, experimental data may be needed to determine the bulk modulus of a gas under specific conditions.
It is also important to note that the required bulk modulus of gases may vary depending on the specific gas being used. For example, some gases, such as hydrogen and helium, have a higher bulk modulus than other gases, such as nitrogen and oxygen, due to differences in their molecular properties.
Overall, determining the required bulk modulus of gases involves careful consideration of the specific application and system requirements, as well as a solid understanding of the properties and behavior of gases under different conditions.
Nomenclature of Bulk modulus of gases
The bulk modulus of gases is typically represented by the symbol K or B. The units of bulk modulus are usually expressed in pressure units, such as pascals (Pa) or pounds per square inch (psi).
In some cases, the bulk modulus may also be expressed in terms of the compressibility of the gas, which is the reciprocal of the bulk modulus. The compressibility is typically represented by the symbol β and has units of inverse pressure, such as Pa^-1 or psi^-1.
The bulk modulus and compressibility of gases can also be influenced by other factors, such as the temperature and pressure of the gas, the presence of impurities or contaminants, and the composition and molecular properties of the gas. As a result, it is important to carefully specify the conditions under which the bulk modulus or compressibility is being measured or calculated.
Case Study on Bulk modulus of gases
One example of a case study on the bulk modulus of gases is in the design and operation of gas pipelines. Gas pipelines are used to transport natural gas, hydrogen, and other gases over long distances, and the bulk modulus of the gas can affect the transmission of pressure and the ability of the pipeline to maintain a constant flow rate.
In the design of gas pipelines, the bulk modulus of the gas is an important factor in determining the required pipeline diameter and wall thickness, as well as the pumping rate and pressure required to maintain a constant flow rate. The bulk modulus of the gas can also affect the response time of the pipeline to changes in pressure or flow rate, which can impact the efficiency and safety of the system.
In one study, researchers investigated the effects of gas compressibility on the performance of a high-pressure natural gas pipeline. They used mathematical models to simulate the behavior of the gas under different pressure and temperature conditions, and calculated the bulk modulus and compressibility of the gas based on its molecular properties.
The results showed that the bulk modulus and compressibility of the gas varied significantly depending on the pressure and temperature of the gas, and that the compressibility had a significant impact on the response time of the pipeline to changes in pressure. The researchers also found that the pipeline diameter and wall thickness needed to be increased in order to accommodate the compressibility of the gas, which increased the cost and complexity of the pipeline design.
Overall, this case study highlights the importance of considering the bulk modulus and compressibility of gases in the design and operation of gas pipelines, and the need for accurate modeling and measurement of these properties under a range of conditions. By carefully considering the effects of gas compressibility, engineers and scientists can design more efficient, reliable, and cost-effective gas transmission systems.
White paper on Bulk modulus of gases
Here is a brief white paper on the bulk modulus of gases:
Introduction:
The bulk modulus of gases is a fundamental property that describes the compressibility of a gas under pressure. It is a measure of the resistance of a gas to a change in volume when subjected to an applied pressure. The bulk modulus of gases plays an important role in various fields, such as mechanical and chemical engineering, physics, and materials science.
Measurement:
The bulk modulus of gases can be measured using various experimental techniques, such as acoustic measurements, density measurements, and compression tests. These methods involve subjecting the gas to a known pressure and measuring the resulting change in volume. The bulk modulus can then be calculated using the formula K = -V(ΔP/ΔV), where K is the bulk modulus, V is the volume of the gas, and ΔP and ΔV are the changes in pressure and volume, respectively.
Properties:
The bulk modulus of gases is influenced by several factors, such as the molecular properties of the gas, the pressure and temperature conditions, and the presence of impurities or contaminants. Generally, gases with higher molecular weights and stronger intermolecular forces have higher bulk moduli than gases with lower molecular weights and weaker intermolecular forces. The bulk modulus of gases also decreases with increasing temperature and pressure, and can be affected by the presence of impurities or contaminants.
Applications:
The bulk modulus of gases is important in various applications, such as the design and operation of gas compressors, pneumatic systems, shock absorbers, gas storage tanks, and gas pipelines. In these applications, the bulk modulus of the gas is used to determine the required pumping rate and pressure, the response time of the system, and the stability and accuracy of the pressure or flow control. The bulk modulus of gases is also used in the study of fluid dynamics and the behavior of gases under different pressure and temperature conditions, and in the design and synthesis of porous materials for gas storage and separation.
Conclusion:
The bulk modulus of gases is a key property that describes the compressibility of a gas under pressure. It is influenced by several factors, such as the molecular properties of the gas, the pressure and temperature conditions, and the presence of impurities or contaminants. The bulk modulus of gases is important in various applications, such as the design and operation of gas transmission systems and the study of fluid dynamics. Accurate measurement and modeling of the bulk modulus of gases is critical for the efficient, reliable, and cost-effective operation of gas-based technologies.
