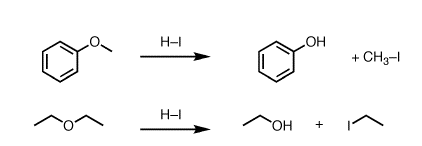
C-O bond cleavage reactions involve breaking the bond between a carbon atom and an oxygen atom in a molecule. These reactions can occur through a variety of mechanisms, including homolytic cleavage, heterolytic cleavage, and acid-catalyzed cleavage.
Homolytic cleavage occurs when the bond between carbon and oxygen is broken evenly, with each atom receiving one of the two electrons that make up the bond. This reaction is often initiated by the input of energy in the form of heat or light.
Heterolytic cleavage occurs when one atom in the bond receives both of the electrons that make up the bond, resulting in the formation of two ions. This reaction often occurs in the presence of a catalyst, such as an acid or a base.
Acid-catalyzed cleavage involves the use of an acid to facilitate the breaking of the C-O bond. This reaction is often used in organic synthesis to break apart molecules and create new ones.
Some examples of C-O bond cleavage reactions include the hydrolysis of an ester, in which the C-O bond is broken by the addition of water in the presence of an acid catalyst, and the oxidation of an alcohol, in which the C-O bond is broken by the removal of hydrogen from the alcohol molecule.
What is Required C-O bond cleavage reactions
The requirements for C-O bond cleavage reactions can vary depending on the specific reaction and mechanism involved. However, in general, some common requirements for C-O bond cleavage reactions include:
- Energy input: In many cases, breaking the C-O bond requires an input of energy. This can be provided by heat, light, or other forms of activation energy.
- Presence of a catalyst: Some C-O bond cleavage reactions require the presence of a catalyst, such as an acid or a base, to facilitate the reaction. The catalyst can help to lower the activation energy required for the reaction to occur.
- Appropriate reaction conditions: The reaction conditions, such as temperature and pressure, need to be appropriate for the specific reaction being performed. For example, some reactions may require high temperatures or high pressure to proceed.
- Appropriate starting materials: The starting materials for the reaction need to have the appropriate functional groups and structures to enable C-O bond cleavage. For example, an alcohol or an ester may be required for certain reactions to occur.
- Reaction time: The reaction may require a certain amount of time to proceed and reach completion. This can vary depending on the specific reaction and mechanism involved.
Overall, C-O bond cleavage reactions require careful consideration of reaction conditions and appropriate starting materials to enable the desired reaction to occur.
When is Required C-O bond cleavage reactions
C-O bond cleavage reactions are important in a wide range of fields, including organic chemistry, biochemistry, and materials science. Some examples of when C-O bond cleavage reactions may be required include:
- Organic synthesis: C-O bond cleavage reactions are commonly used in organic synthesis to create new molecules with specific functional groups. For example, the hydrolysis of an ester can be used to produce a carboxylic acid and an alcohol, while the oxidation of an alcohol can be used to produce a ketone or an aldehyde.
- Polymer chemistry: C-O bond cleavage reactions are important in polymer chemistry, where they are used to break down and recycle polymers. For example, the depolymerization of PET (polyethylene terephthalate) involves C-O bond cleavage to break down the polymer into its constituent monomers.
- Biochemistry: C-O bond cleavage reactions are important in many biological processes, such as the breakdown of carbohydrates and lipids for energy. Enzymes catalyze C-O bond cleavage reactions in these processes to facilitate the breakdown of these molecules into smaller, more usable forms.
- Materials science: C-O bond cleavage reactions are important in materials science for the synthesis of new materials with specific properties. For example, the functionalization of carbon nanotubes with oxygen-containing groups involves C-O bond cleavage to create new functional groups on the nanotube surface.
Overall, C-O bond cleavage reactions are an important tool for chemists and researchers in a variety of fields to synthesize new compounds, break down and recycle materials, and understand biological processes.
Where is Required C-O bond cleavage reactions
C-O bond cleavage reactions are required in various areas of science, including:
- Organic chemistry: In organic chemistry, C-O bond cleavage reactions are commonly used in the synthesis of complex molecules. For example, the cleavage of esters, ethers, and acetals is a fundamental step in the synthesis of many natural products, pharmaceuticals, and other organic compounds.
- Polymer chemistry: C-O bond cleavage reactions are important in the recycling and degradation of polymers. For instance, the depolymerization of cellulose and lignocellulosic biomass involves the cleavage of the glycosidic bond, which is a C-O bond connecting the glucose units.
- Biochemistry: C-O bond cleavage reactions are critical in the metabolic pathways of living organisms. For example, the breakdown of glucose during glycolysis and the Krebs cycle involves several C-O bond cleavage reactions that ultimately produce ATP, which is the energy currency of the cell.
- Materials science: In materials science, C-O bond cleavage reactions are utilized for the functionalization of surfaces and interfaces. For instance, the incorporation of oxygen-containing functional groups on the surface of graphene involves C-O bond cleavage, which improves the dispersibility and solubility of graphene in various solvents.
Overall, C-O bond cleavage reactions are widely required in various areas of chemistry, materials science, and biochemistry for the synthesis of new compounds, degradation of materials, and understanding of complex biological processes.
How is Required C-O bond cleavage reactions
C-O bond cleavage reactions can occur through several mechanisms, depending on the specific reaction and the type of bond being cleaved. Here are some examples:
- Hydrolysis: One of the most common methods for C-O bond cleavage is hydrolysis, which involves the addition of water to the bond, resulting in the cleavage of the C-O bond. For example, the hydrolysis of an ester involves the cleavage of the C-O bond between the carbonyl group and the alcohol group.
- Reduction: Reduction reactions involve the gain of electrons, which can be used to cleave C-O bonds. For example, the reduction of a ketone or an aldehyde with a reducing agent such as sodium borohydride or lithium aluminum hydride can result in the cleavage of the C-O bond to produce a primary or a secondary alcohol.
- Oxidation: Oxidation reactions involve the loss of electrons, which can also be used to cleave C-O bonds. For example, the oxidation of an alcohol with an oxidizing agent such as Jones reagent or potassium permanganate can result in the cleavage of the C-O bond to produce a ketone or an aldehyde.
- Enzymatic reactions: Many C-O bond cleavage reactions in biological systems are catalyzed by enzymes. For example, the cleavage of the C-O bond in glucose during glycolysis is catalyzed by several enzymes, including aldolase and triose phosphate isomerase.
Overall, the mechanism of C-O bond cleavage depends on the specific reaction and the type of bond being cleaved, and can involve a variety of chemical and enzymatic reactions.
Production of C-O bond cleavage reactions
The production of C-O bond cleavage reactions depends on the specific reaction and the desired outcome. Here are some examples of how C-O bond cleavage reactions can be produced:
- Chemical reactions: Chemical reactions can be used to produce C-O bond cleavage, as described in the previous answer. For example, the hydrolysis of an ester can be achieved by the addition of water and an acid or base catalyst.
- Enzymatic reactions: Enzymes can be used to catalyze C-O bond cleavage reactions in biological systems. For example, the enzyme cellulase can cleave the C-O bond in cellulose, which is a major component of plant cell walls.
- Thermal reactions: C-O bond cleavage reactions can also be produced by thermal means, such as heating or pyrolysis. For example, the pyrolysis of lignin, a component of wood, can result in the cleavage of C-O bonds, producing various chemicals and biofuels.
- Photoreactions: C-O bond cleavage can also be produced by photochemical reactions, where light energy is used to initiate the reaction. For example, the photocatalytic cleavage of the C-O bond in water molecules using sunlight can produce hydrogen and oxygen, which are used in fuel cells.
Overall, the production of C-O bond cleavage reactions depends on the desired outcome and the specific reaction mechanism involved. Various chemical, enzymatic, thermal, and photoreactions can be used to produce C-O bond cleavage reactions in different fields, including organic chemistry, biochemistry, materials science, and environmental science.
Case Study on C-O bond cleavage reactions
One case study where C-O bond cleavage reactions are important is in the production of biofuels from lignocellulosic biomass. Lignocellulosic biomass, which includes agricultural and forestry residues, is a promising source of renewable energy because it is abundant and does not compete with food crops. The production of biofuels from lignocellulosic biomass involves several C-O bond cleavage reactions, which are essential for breaking down the complex biomass into simple sugars that can be fermented into biofuels.
The main component of lignocellulosic biomass is cellulose, which is a polysaccharide made up of glucose units connected by C-O bonds. The first step in the production of biofuels from lignocellulosic biomass is the pretreatment of the biomass, which involves the cleavage of C-O bonds to break down the structure of cellulose and make it more accessible to enzymes. Pretreatment can be achieved using various methods, including acid hydrolysis, steam explosion, and ammonia fiber expansion.
After pretreatment, the cellulose is hydrolyzed into simple sugars using enzymes, which catalyze the cleavage of the C-O bonds in cellulose. The resulting sugars are then fermented into biofuels such as ethanol or butanol. The fermentation process involves the cleavage of C-O bonds in the sugars, resulting in the production of ethanol or butanol and carbon dioxide.
The production of biofuels from lignocellulosic biomass requires multiple C-O bond cleavage reactions, which are critical for breaking down the complex biomass into simple sugars that can be used as feedstock for biofuels. This process is still being optimized, and researchers are exploring various pretreatment and enzyme technologies to increase the efficiency and yield of biofuel production from lignocellulosic biomass.
White paper on C-O bond cleavage reactions
Introduction:
C-O bond cleavage reactions are an important class of chemical reactions that involve the breaking of a covalent bond between a carbon atom and an oxygen atom. These reactions have numerous applications in organic synthesis, biochemistry, materials science, and environmental science. In this white paper, we will provide an overview of C-O bond cleavage reactions, including their mechanisms, applications, and challenges.
Mechanisms of C-O Bond Cleavage Reactions:
C-O bond cleavage reactions can occur through several mechanisms, depending on the specific reaction and the type of bond being cleaved. One of the most common methods for C-O bond cleavage is hydrolysis, which involves the addition of water to the bond, resulting in the cleavage of the C-O bond. For example, the hydrolysis of an ester involves the cleavage of the C-O bond between the carbonyl group and the alcohol group.
Reduction and oxidation reactions also involve C-O bond cleavage. Reduction reactions involve the gain of electrons, which can be used to cleave C-O bonds. For example, the reduction of a ketone or an aldehyde with a reducing agent such as sodium borohydride or lithium aluminum hydride can result in the cleavage of the C-O bond to produce a primary or a secondary alcohol. On the other hand, oxidation reactions involve the loss of electrons, which can also be used to cleave C-O bonds. For example, the oxidation of an alcohol with an oxidizing agent such as Jones reagent or potassium permanganate can result in the cleavage of the C-O bond to produce a ketone or an aldehyde.
Enzymatic reactions are another mechanism for C-O bond cleavage reactions. Many C-O bond cleavage reactions in biological systems are catalyzed by enzymes. For example, the cleavage of the C-O bond in glucose during glycolysis is catalyzed by several enzymes, including aldolase and triose phosphate isomerase.
Applications of C-O Bond Cleavage Reactions:
C-O bond cleavage reactions have numerous applications in various fields, including organic synthesis, biochemistry, materials science, and environmental science. Some of the most important applications of C-O bond cleavage reactions are:
- Synthesis of organic compounds: C-O bond cleavage reactions are widely used in organic synthesis to construct complex molecules. For example, the cleavage of an ether bond can be used to produce an alcohol and an alkyl halide, which can then be used in further synthetic transformations.
- Production of biofuels: C-O bond cleavage reactions are essential in the production of biofuels from lignocellulosic biomass. The cleavage of C-O bonds in cellulose is critical for breaking down the complex biomass into simple sugars that can be fermented into biofuels such as ethanol or butanol.
- Drug discovery and development: C-O bond cleavage reactions are frequently used in drug discovery and development to modify or cleave specific bonds in a molecule. For example, the cleavage of a C-O bond in a drug molecule can result in the formation of a reactive intermediate that can interact with a target protein or enzyme.
Challenges in C-O Bond Cleavage Reactions:
Although C-O bond cleavage reactions have numerous applications, they also present several challenges. One of the main challenges is the selectivity of the reaction. C-O bond cleavage reactions can result in the cleavage of multiple bonds in a molecule, making it difficult to control the reaction and obtain the desired product. Another challenge is the use of toxic or hazardous reagents, particularly in industrial applications. The development of more selective and environmentally friendly C-O bond cleavage reactions is an ongoing area of research.
Conclusion:
C-O bond cleavage reactions are a vital class of chemical reactions with widespread applications in various fields. They can occur through different mechanisms, including hydrolysis, reduction, oxidation, and enzymatic reactions. These reactions have applications in organic synthesis, biochemistry, materials science, and environmental science, including the production of biofuels, drug discovery and development, and synthesis of complex molecules. However, C-O bond cleavage reactions also present challenges, such as selectivity issues and the use of hazardous reagents. The development of more selective and environmentally friendly C-O bond cleavage reactions is an active area of research. Overall, understanding the mechanisms, applications, and challenges of C-O bond cleavage reactions is essential for advancing chemistry and related fields.
