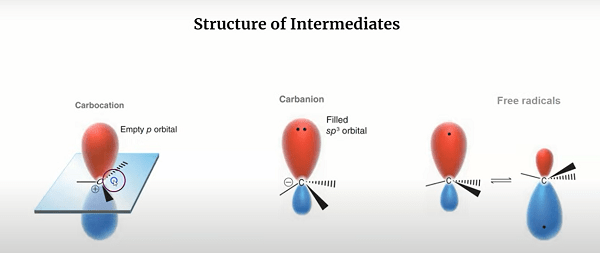
Carbanions and free radicals are two types of reactive intermediates that are commonly encountered in organic chemistry.
A carbanion is an anionic species with a negatively charged carbon atom. It is formed when a carbon atom gains an electron pair and becomes negatively charged. Carbanions are typically very reactive and are often involved in nucleophilic substitution and elimination reactions. They can be stabilized by electron-withdrawing substituents, such as halogens or carbonyl groups, that help to disperse the negative charge and reduce its reactivity.
Free radicals, on the other hand, are neutral species with an unpaired electron. They are formed when a molecule loses an electron, leaving behind an unpaired electron on an atom. Free radicals are also very reactive and can be involved in a variety of reactions, including radical addition, radical substitution, and radical polymerization. They can be stabilized by resonance, as well as by electron-donating substituents that help to distribute the unpaired electron and reduce its reactivity.
Both carbanions and free radicals are important intermediates in organic chemistry and are used in a variety of synthetic transformations. However, they are also highly reactive and can be difficult to handle, so special precautions are often required when working with these types of species.
What is Required Basic Principles of Organic Chemistry Carbanions and Free radicals
There are several basic principles that are important to understand when working with carbanions and free radicals in organic chemistry:
- Stability: Both carbanions and free radicals are highly reactive, but their stability can be increased through various means. For carbanions, this can involve the use of electron-withdrawing substituents that help to stabilize the negative charge. For free radicals, stability can be achieved through resonance and the use of electron-donating substituents that help to distribute the unpaired electron.
- Reactivity: While both carbanions and free radicals are highly reactive, they exhibit different reactivities due to their electronic structures. Carbanions are strong nucleophiles that can participate in nucleophilic substitution and elimination reactions, while free radicals are typically involved in radical addition, substitution, and polymerization reactions.
- Formation: Both carbanions and free radicals can be formed through a variety of methods, including through the use of strong bases or nucleophiles, or through the use of radical initiators such as peroxides or photolysis. It is important to understand the conditions under which these intermediates are formed in order to predict their behavior in subsequent reactions.
- Kinetics: The kinetics of reactions involving carbanions and free radicals can be complex and are influenced by a variety of factors, including temperature, solvent, and concentration. Understanding these kinetic factors is important in order to design and optimize synthetic reactions that involve these intermediates.
By understanding these basic principles of carbanions and free radicals, organic chemists can better predict their behavior and design synthetic routes that take advantage of their reactivity.
When is Required Basic Principles of Organic Chemistry Carbanions and Free radicals
The basic principles of carbanions and free radicals in organic chemistry are required in a variety of situations, including:
- Organic Synthesis: Carbanions and free radicals are important intermediates in many synthetic transformations, such as nucleophilic substitution, elimination, addition, and polymerization reactions. Understanding the principles that govern their reactivity and stability is essential for designing efficient and selective synthetic routes.
- Mechanistic Studies: Carbanions and free radicals are often involved in complex reaction mechanisms, and understanding their behavior is essential for understanding the overall reaction pathway. Detailed studies of the kinetics and thermodynamics of reactions involving these intermediates can provide insight into the underlying mechanisms.
- Biochemistry: Carbanions and free radicals are also important in biochemistry, where they play key roles in enzymatic catalysis and cellular signaling pathways. Understanding the principles that govern their behavior is essential for understanding the mechanisms of biological processes.
- Materials Science: Carbanions and free radicals can also be used in materials science applications, such as in the synthesis of conductive polymers or in the formation of thin films. Understanding their reactivity and stability is important for designing materials with desired properties.
Overall, a thorough understanding of the basic principles of carbanions and free radicals is essential for a wide range of applications in organic chemistry and related fields.
Where is Required Basic Principles of Organic Chemistry Carbanions and Free radicals
The basic principles of carbanions and free radicals in organic chemistry are required in a variety of settings, including:
- Academic Research: Organic chemists in academia study carbanions and free radicals as key intermediates in various synthetic reactions and mechanisms. Understanding their properties is essential for developing new methods for the synthesis of organic molecules and for elucidating reaction mechanisms.
- Pharmaceutical Industry: The pharmaceutical industry utilizes carbanions and free radicals in the synthesis of drug molecules. Understanding their reactivity and stability is essential for developing efficient synthetic routes and for ensuring the safety and efficacy of drug products.
- Chemical Industry: The chemical industry uses carbanions and free radicals in the production of various industrial chemicals, such as plastics, polymers, and solvents. Understanding their properties is important for optimizing production processes and developing new applications for these materials.
- Materials Science: Carbanions and free radicals are also important in materials science, where they are used in the synthesis of materials with desired electronic and optical properties. Understanding their reactivity and stability is important for designing new materials with specific properties.
Overall, the basic principles of carbanions and free radicals are required in a wide range of settings in organic chemistry, including academic research, pharmaceuticals, chemical industry, and materials science.
How is Required Basic Principles of Organic Chemistry Carbanions and Free radicals
The basic principles of carbanions and free radicals in organic chemistry are typically learned through a combination of textbook study, lecture-based teaching, and laboratory experience.
In a typical organic chemistry course, students learn about the principles of reactivity and stability of carbanions and free radicals through the study of reaction mechanisms, as well as through the use of computational chemistry tools that can predict their behavior. Students may also learn about the conditions under which carbanions and free radicals can be formed, and the factors that influence their reactivity and kinetics.
Laboratory experience is also essential for learning about carbanions and free radicals in organic chemistry. In the laboratory, students can observe the behavior of these intermediates in real-time and gain hands-on experience with techniques for handling and manipulating these highly reactive species. They may also learn about the use of spectroscopic techniques such as NMR and EPR for characterizing these intermediates and monitoring their behavior in real-time.
Overall, a combination of textbook study, lecture-based teaching, and laboratory experience is required to gain a thorough understanding of the basic principles of carbanions and free radicals in organic chemistry.
Nomenclature of Basic Principles of Organic Chemistry Carbanions and Free radicals
Carbanions and free radicals are both classified as organic chemical species that have unique nomenclature rules based on their electronic configuration and reactivity.
- Carbanions: Carbanions are negatively charged species with a carbon atom that has an unshared pair of electrons. They are named by adding the suffix “-ide” to the name of the parent hydrocarbon and adding the word “carbanion” after the suffix. For example, the carbanion derived from ethane is named ethanide carbanion. If the carbanion is derived from a substituted hydrocarbon, the substituent is named first and then followed by the word “carbanion.” For example, the carbanion derived from 2-methylpropane is named 2-methylpropanide carbanion.
- Free Radicals: Free radicals are species that have an unpaired electron in their outermost valence shell. They are named using the suffix “-yl” attached to the name of the parent hydrocarbon. For example, the methyl free radical is named methyl radical. If the free radical is derived from a substituted hydrocarbon, the substituent is named first and then followed by the suffix “-yl.” For example, the free radical derived from 2-methylpropane is named 2-methylpropyl radical.
It is important to note that the nomenclature of carbanions and free radicals can become more complex when dealing with more complex organic molecules, and the use of IUPAC rules is recommended for naming these species accurately.
Case Study on Basic Principles of Organic Chemistry Carbanions and Free radicals
One example of the use of carbanions and free radicals in organic chemistry can be seen in the synthesis of drug molecules. One such drug is the antiviral drug acyclovir, which is used to treat herpes infections.
Acyclovir is synthesized from guanine, which is a naturally occurring nucleic acid base. The first step in the synthesis involves the formation of a carbanion intermediate by treating guanine with sodium hydride (NaH) in an aprotic solvent such as dimethyl sulfoxide (DMSO). The carbanion is formed at the C-6 position of guanine due to the high electronegativity of the adjacent nitrogen atom, which stabilizes the negative charge on the carbanion.
Once the carbanion is formed, it reacts with chloroacetic acid to form a new carbon-carbon bond, resulting in the formation of a new six-membered ring. The intermediate that is formed is then treated with triethylamine, which acts as a base to remove the proton from the alpha-carbon, resulting in the formation of a free radical intermediate. This free radical then reacts with acetyl chloride to form the acyclovir molecule.
Overall, the synthesis of acyclovir demonstrates the important role that carbanions and free radicals play in organic chemistry, particularly in the synthesis of complex organic molecules. Understanding the properties of these intermediates is essential for developing efficient synthetic routes and for ensuring the safety and efficacy of drug products.
White paper on Basic Principles of Organic Chemistry Carbanions and Free radicals
Introduction:
Organic chemistry is the study of carbon-based compounds and their properties, reactivity, and behavior. Two important intermediates in organic chemistry are carbanions and free radicals. Carbanions are species with a negatively charged carbon atom that possesses an unshared pair of electrons, while free radicals have an unpaired electron in their outermost valence shell. This white paper aims to provide an overview of the basic principles of carbanions and free radicals in organic chemistry, their properties, and their reactivity.
Properties of Carbanions:
Carbanions are generally highly reactive species due to their negative charge and unshared electron pair on the carbon atom. The stability of carbanions depends on the electronegativity of the adjacent atoms and the steric effects of nearby groups. The greater the electronegativity of the adjacent atoms, the more stable the carbanion. For example, in the formation of acyclovir from guanine, the carbanion intermediate is formed at the C-6 position of guanine due to the high electronegativity of the adjacent nitrogen atom, which stabilizes the negative charge on the carbanion.
Carbanions can also undergo nucleophilic reactions, where they donate a pair of electrons to an electrophilic species. For example, in the synthesis of ketones and aldehydes, a carbanion intermediate is formed when a Grignard reagent is treated with a carbonyl compound. The carbanion attacks the electrophilic carbon in the carbonyl group, resulting in the formation of a new carbon-carbon bond.
Properties of Free Radicals:
Free radicals are highly reactive species due to their unpaired electron in their outermost valence shell. Free radicals can react with other molecules and can initiate chain reactions, leading to the formation of new products. The reactivity of free radicals is influenced by factors such as temperature, pressure, and the presence of other molecules.
Free radicals can undergo a variety of reactions, including addition, abstraction, and fragmentation reactions. Addition reactions occur when the free radical adds to a double bond, resulting in the formation of a new carbon-carbon bond. Abstraction reactions occur when the free radical removes a hydrogen atom from a molecule, resulting in the formation of a new free radical intermediate. Fragmentation reactions occur when the free radical breaks apart into smaller fragments.
Applications of Carbanions and Free Radicals:
Carbanions and free radicals are important intermediates in many organic reactions and are used in a variety of applications. For example, carbanions are used in the synthesis of complex organic molecules, such as drugs and natural products. Free radicals are used in polymerization reactions, where they initiate the formation of new polymers.
Conclusion:
Carbanions and free radicals are important intermediates in organic chemistry, and their properties and reactivity are essential for understanding the behavior of complex organic molecules. The stability and reactivity of these species depend on factors such as electronegativity, steric effects, temperature, and pressure. Understanding the behavior of carbanions and free radicals is essential for developing efficient synthetic routes and for ensuring the safety and efficacy of drug products and other organic compounds.
