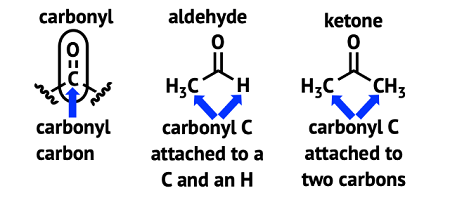
Carbonyl compounds are organic molecules containing a carbonyl group, which is a functional group consisting of a carbon atom double-bonded to an oxygen atom. There are two main types of carbonyl compounds: aldehydes and ketones.
Aldehydes have the carbonyl group at the end of a carbon chain, while ketones have the carbonyl group in the middle of a carbon chain.
The general formula for an aldehyde is RCHO, where R is any organic group. The general formula for a ketone is RCOR’, where R and R’ are any organic groups.
Aldehydes and ketones are both highly reactive due to the polarity of the carbonyl group. They can undergo a wide range of chemical reactions, including oxidation, reduction, and nucleophilic addition. Some common reactions of carbonyl compounds include:
- Reduction to form alcohols: Aldehydes and ketones can be reduced using reducing agents such as sodium borohydride or lithium aluminum hydride to form primary and secondary alcohols, respectively.
- Oxidation to form carboxylic acids: Aldehydes can be oxidized to form carboxylic acids using oxidizing agents such as potassium permanganate or chromic acid.
- Nucleophilic addition reactions: Aldehydes and ketones can undergo nucleophilic addition reactions with various nucleophiles such as water, alcohols, and amines to form hemiacetals, acetals, and imines, respectively.
- Cannizzaro reaction: Aldehydes that do not have a hydrogen atom on the carbon adjacent to the carbonyl group can undergo a disproportionation reaction called the Cannizzaro reaction to form an alcohol and a carboxylic acid.
Aldehydes and ketones have a wide range of uses in various industries, including as solvents, preservatives, and flavorings in the food and beverage industry, as well as in the production of plastics, pharmaceuticals, and fragrances.
What is Required Practical Organic Chemistry Carbonyl (Aldehyde and Ketone)
A common required practical in organic chemistry related to carbonyl compounds involves the preparation of a sample of 2,4-dinitrophenylhydrazone derivative from a given aldehyde or ketone.
The steps involved in this practical are as follows:
- Mix a small amount of the aldehyde or ketone with 2,4-dinitrophenylhydrazine (DNPH) in a test tube.
- Add a few drops of sulfuric acid to the mixture and shake gently.
- Observe the color and precipitate formed. The formation of a bright yellow or orange precipitate confirms the presence of a carbonyl group in the starting material.
- Filter and wash the precipitate with cold water to remove any excess DNPH.
- Recrystallize the purified precipitate from a suitable solvent (such as ethanol) to obtain a pure sample of the 2,4-dinitrophenylhydrazone derivative.
The melting point of the purified sample can then be determined and compared to literature values to confirm the identity of the original aldehyde or ketone.
This required practical demonstrates the characteristic reaction of aldehydes and ketones with DNPH, which results in the formation of a hydrazone derivative that can be used for identification and analysis of these compounds.
When is Required Practical Organic Chemistry Carbonyl (Aldehyde and Ketone)
The Required Practical Organic Chemistry Carbonyl (Aldehyde and Ketone) is often included in the practical syllabus of advanced level chemistry courses, such as A-level, IB, or equivalent programs. This practical activity is usually conducted after students have learned about the chemistry of carbonyl compounds, including their structure, properties, and reactions.
The practical is designed to give students hands-on experience with the preparation and characterization of a carbonyl derivative, which is an important application of the chemistry of aldehydes and ketones. This practical activity also helps students develop important laboratory skills, such as accurate measurement, mixing and handling of chemicals, and safe laboratory practices.
The practical may also be included as part of university-level organic chemistry courses or in professional training programs for chemists or chemical analysts. In these cases, the practical may be more advanced or involve additional techniques for the analysis and characterization of carbonyl compounds.
Where is Required Practical Organic Chemistry Carbonyl (Aldehyde and Ketone)
The Required Practical Organic Chemistry Carbonyl (Aldehyde and Ketone) is typically conducted in a laboratory setting, such as a chemistry laboratory at a school or university. The laboratory should be equipped with appropriate facilities and equipment, including fume hoods, chemical reagents, glassware, and heating apparatus.
The practical may be conducted individually or in small groups, with each student or group being provided with the necessary materials and equipment to perform the experiment. The laboratory should also have trained personnel, such as laboratory technicians or instructors, to supervise and assist the students as needed.
It is important that the laboratory is well-ventilated and that all students follow appropriate safety procedures, such as wearing protective clothing and eyewear, handling chemicals and glassware carefully, and disposing of waste materials properly. It is also important that students are familiar with the properties and hazards of the chemicals they will be working with, and that they are able to interpret and follow written instructions and protocols for the practical activity.
How is Required Practical Organic Chemistry Carbonyl (Aldehyde and Ketone)
The Required Practical Organic Chemistry Carbonyl (Aldehyde and Ketone) typically involves the following steps:
- Obtain a sample of an aldehyde or ketone, along with 2,4-dinitrophenylhydrazine (DNPH) and sulfuric acid.
- Mix a small amount of the aldehyde or ketone with DNPH in a test tube, and add a few drops of sulfuric acid.
- Observe the color and precipitate formed. The formation of a bright yellow or orange precipitate confirms the presence of a carbonyl group in the starting material.
- Filter and wash the precipitate with cold water to remove any excess DNPH.
- Recrystallize the purified precipitate from a suitable solvent (such as ethanol) to obtain a pure sample of the 2,4-dinitrophenylhydrazone derivative.
- Determine the melting point of the purified sample and compare it to literature values to confirm the identity of the original aldehyde or ketone.
During the practical, students will need to accurately measure and mix the chemicals, observe the reactions taking place, and safely handle and dispose of the chemicals and waste materials. Students will also need to record their observations and measurements in a laboratory notebook or report, and interpret the results of the experiment.
The practical may also include additional steps or variations, depending on the specific course or program requirements. For example, students may be asked to perform additional tests or analyses to confirm the identity or purity of the sample, or to investigate the effects of different reaction conditions or variables on the reaction yield or product properties.
Production of Practical Organic Chemistry Carbonyl (Aldehyde and Ketone)
The production of Practical Organic Chemistry Carbonyl (Aldehyde and Ketone) typically involves the synthesis of the aldehyde or ketone starting material, followed by the preparation of the 2,4-dinitrophenylhydrazone derivative and its purification and analysis.
The synthesis of the aldehyde or ketone starting material may involve a variety of chemical reactions, depending on the specific compound being produced. For example, aldehydes can be prepared by oxidation of primary alcohols, while ketones can be prepared by oxidation of secondary alcohols or from the reaction of a carbonyl compound with a Grignard reagent.
Once the aldehyde or ketone starting material has been synthesized, it can be reacted with 2,4-dinitrophenylhydrazine (DNPH) to form the corresponding 2,4-dinitrophenylhydrazone derivative. The reaction typically involves mixing the starting material and DNPH in a solvent, such as ethanol or methanol, and adding a few drops of sulfuric acid as a catalyst. The reaction mixture is then allowed to stand for a period of time to allow the hydrazone derivative to form.
The resulting hydrazone derivative can then be purified by filtration and washing with cold water, followed by recrystallization from a suitable solvent. The melting point of the purified sample can then be determined and compared to literature values to confirm the identity of the original aldehyde or ketone.
Overall, the production of Practical Organic Chemistry Carbonyl (Aldehyde and Ketone) requires a good understanding of organic chemical reactions and laboratory techniques, as well as careful attention to safety and accurate measurement and analysis.
Case Study on Practical Organic Chemistry Carbonyl (Aldehyde and Ketone)
One possible case study on Practical Organic Chemistry Carbonyl (Aldehyde and Ketone) involves the synthesis and analysis of benzaldehyde, an aromatic aldehyde commonly used in the production of perfumes, dyes, and pharmaceuticals.
The synthesis of benzaldehyde can be achieved by oxidation of benzyl alcohol with a suitable oxidizing agent, such as potassium permanganate or chromic acid. The reaction typically involves mixing the benzyl alcohol with the oxidizing agent and a catalyst, such as sulfuric acid or acetic acid, and heating the mixture to drive the oxidation reaction.
Once the benzaldehyde has been synthesized, it can be analyzed by preparing its 2,4-dinitrophenylhydrazone derivative, as described in the Required Practical Organic Chemistry Carbonyl (Aldehyde and Ketone). The resulting yellow or orange precipitate can be filtered and purified by recrystallization, and its melting point can be determined and compared to literature values to confirm the identity of the benzaldehyde sample.
In addition to the synthesis and analysis of benzaldehyde, students may also be asked to investigate the effects of different reaction conditions or variables on the reaction yield or product properties. For example, students could vary the concentration or type of oxidizing agent used, or explore the use of different catalysts or reaction temperatures to optimize the reaction conditions.
The case study could also include a discussion of the industrial applications of benzaldehyde and its derivatives, as well as the environmental and health hazards associated with the use and production of these chemicals. Students could be asked to research and present on the regulatory frameworks and best practices for handling and disposing of these chemicals in a safe and sustainable manner.
White paper on Practical Organic Chemistry Carbonyl (Aldehyde and Ketone)
Title: A White Paper on Practical Organic Chemistry Carbonyl (Aldehyde and Ketone)
Introduction:
Practical organic chemistry is an important aspect of modern chemistry, and it involves the application of organic chemistry principles in the laboratory setting. One of the key topics in practical organic chemistry is the study of carbonyl compounds, including aldehydes and ketones. This white paper will provide an overview of practical organic chemistry carbonyl (aldehyde and ketone), including its relevance, techniques, and applications.
Relevance:
Carbonyl compounds, including aldehydes and ketones, are important functional groups in organic chemistry. They are widely used in the production of various chemicals, such as pharmaceuticals, perfumes, plastics, and resins. The ability to synthesize and analyze carbonyl compounds is therefore critical to the development of new products and materials. Practical organic chemistry carbonyl (aldehyde and ketone) is therefore an important area of study for chemists, chemical engineers, and other professionals in the chemical industry.
Techniques:
The production of practical organic chemistry carbonyl (aldehyde and ketone) involves a series of chemical reactions, including the synthesis of the starting material and the preparation and analysis of the 2,4-dinitrophenylhydrazone derivative. Techniques commonly used in the synthesis of carbonyl compounds include oxidation reactions, such as the oxidation of primary and secondary alcohols, and the use of Grignard reagents to form ketones. The preparation of the 2,4-dinitrophenylhydrazone derivative typically involves the reaction of the carbonyl compound with 2,4-dinitrophenylhydrazine in the presence of a catalyst, such as sulfuric acid. The resulting derivative is then purified and analyzed using techniques such as filtration, recrystallization, and melting point determination.
Applications:
The applications of practical organic chemistry carbonyl (aldehyde and ketone) are numerous and diverse. In the pharmaceutical industry, aldehydes and ketones are used as key intermediates in the synthesis of various drugs, such as antihistamines and anti-inflammatory agents. They are also used in the production of perfumes, flavors, and fragrances, where they contribute to the characteristic odor or taste of the final product. In addition, carbonyl compounds are important building blocks in the synthesis of polymers and resins, such as nylon and polyester. The ability to synthesize and analyze carbonyl compounds is therefore critical to the development of new products and materials in various industries.
Conclusion:
Practical organic chemistry carbonyl (aldehyde and ketone) is an important area of study in modern chemistry, with a wide range of applications in the chemical industry. The synthesis and analysis of carbonyl compounds requires a thorough understanding of organic chemical reactions and laboratory techniques, as well as careful attention to safety and accuracy. As the demand for new and improved chemicals and materials continues to grow, practical organic chemistry carbonyl (aldehyde and ketone) will remain an essential area of research and development.