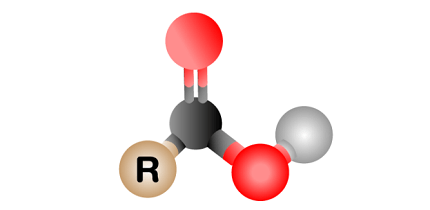
Carboxyl refers to a functional group that consists of a carbon atom that is double-bonded to an oxygen atom, which is also bonded to a hydroxyl (-OH) group. This functional group is commonly found in organic compounds such as carboxylic acids, which are characterized by their acidic properties due to the presence of the carboxyl group. In a carboxylic acid molecule, the carboxyl group is attached to a carbon atom that is also bonded to one or more other atoms or groups, such as hydrogen or alkyl groups. The carboxyl group is important in biochemistry as it is found in many essential biomolecules such as amino acids and fatty acids.
What is Required Practical Organic Chemistry Carboxyl
There are several required practical experiments in organic chemistry that involve carboxyl groups. Here are a few examples:
- Preparation of an organic acid: This experiment involves the preparation of an organic acid, such as ethanoic acid (acetic acid), from a suitable alcohol using oxidizing agents like potassium permanganate or acidified potassium dichromate.
- Titration of an organic acid: In this experiment, you can determine the concentration of an organic acid solution by titrating it against a standard solution of sodium hydroxide. This method is commonly used to determine the acid content of vinegar.
- Synthesis of an ester: Esters are organic compounds formed by the reaction between an organic acid and an alcohol. In this experiment, you can synthesize an ester, such as ethyl ethanoate, by reacting ethanoic acid with ethanol in the presence of a catalyst like concentrated sulfuric acid.
- Hydrolysis of an ester: Ester hydrolysis is a reaction in which an ester is broken down into its component acid and alcohol in the presence of an acid or base. In this experiment, you can hydrolyze an ester, such as ethyl ethanoate, using dilute hydrochloric acid, and then identify the products formed.
These practical experiments are designed to help students understand the properties and reactions of carboxyl groups and organic acids, and how they can be used in various applications, including the synthesis of esters and the determination of acid content in solutions.
When is Required Practical Organic Chemistry Carboxyl
Required practical experiments in organic chemistry that involve carboxyl groups are typically conducted as part of undergraduate-level organic chemistry courses, usually in the second or third year of study. These practicals may be included as part of a larger course or module that covers the properties and reactions of organic compounds, or they may be specifically focused on carboxyl groups and their derivatives. The experiments may be conducted in a laboratory setting, either individually or in small groups, and may be supervised by a lab instructor or teaching assistant. Some of these experiments may also be included in advanced high school chemistry courses, especially those that cover organic chemistry in detail.
Where is Required Practical Organic Chemistry Carboxyl
Required practical experiments in organic chemistry that involve carboxyl groups can be conducted in a laboratory setting. This may be a dedicated chemistry laboratory within a university or college, or it may be a shared laboratory space that is used for multiple science disciplines. In some cases, these experiments may also be conducted in a high school chemistry laboratory, although the facilities and resources available may be more limited.
In a typical laboratory setting, students will have access to all of the equipment and reagents necessary to conduct the experiments safely and accurately. This may include glassware such as beakers, flasks, and test tubes, as well as specialized equipment such as burettes, pipettes, and titration apparatus. In addition, students will typically have access to fume hoods, safety equipment such as gloves and safety glasses, and other resources necessary to ensure that the experiments are conducted safely and effectively.
Overall, the location of required practical experiments in organic chemistry that involve carboxyl groups will depend on the specific course or program, as well as the resources and facilities available to the students.
How is Required Practical Organic Chemistry Carboxyl
The required practical experiments in organic chemistry that involve carboxyl groups typically involve a series of steps that are designed to help students understand the properties and reactions of organic acids, as well as the synthesis and hydrolysis of esters. Here is a general outline of how some of these experiments might be conducted:
- Preparation of an organic acid: Students would start by selecting a suitable alcohol, such as ethanol, and reacting it with an oxidizing agent such as potassium permanganate or acidified potassium dichromate. The reaction would be carried out under controlled conditions, such as in a fume hood, and the resulting organic acid would be purified and characterized using techniques such as melting point determination and infrared spectroscopy.
- Titration of an organic acid: Students would prepare a solution of the organic acid of known concentration and then titrate it against a standard solution of sodium hydroxide. The endpoint of the titration would be determined using a suitable indicator, such as phenolphthalein, and the concentration of the acid solution would be calculated using stoichiometric principles.
- Synthesis of an ester: Students would react the organic acid, such as ethanoic acid, with an alcohol, such as ethanol, in the presence of a catalyst such as concentrated sulfuric acid. The reaction would be monitored and the resulting ester, such as ethyl ethanoate, would be purified and characterized using techniques such as gas chromatography and nuclear magnetic resonance spectroscopy.
- Hydrolysis of an ester: Students would hydrolyze the ester, such as ethyl ethanoate, using dilute hydrochloric acid, and then identify the products formed using techniques such as thin-layer chromatography and infrared spectroscopy.
Throughout these experiments, students would be expected to document their procedures, observations, and results in a laboratory notebook, and to analyze their data and draw conclusions about the properties and reactions of carboxyl groups and their derivatives. They would also be expected to follow proper laboratory safety procedures and protocols, and to work collaboratively with their lab partners and instructors.
Nomenclature of Practical Organic Chemistry Carboxyl
In organic chemistry, carboxyl groups are named using the suffix “-oic acid”. The basic rules for naming carboxylic acids are as follows:
- Find the longest continuous chain of carbon atoms that contains the carboxyl group.
- Number the chain so that the carboxyl carbon atom has the lowest possible number.
- Replace the “-e” ending of the parent alkane name with “-oic acid”.
- If there are other functional groups present in the molecule, they should be named and located using appropriate prefixes and numbers.
For example, the compound with the formula CH3CH2COOH contains a carboxyl group and is named ethanoic acid. The parent alkane is ethane, the carboxyl carbon atom has the lowest possible number (1), and the “-e” ending is replaced with “-oic acid”.
If there are multiple carboxyl groups in the molecule, the prefix “di-” (two), “tri-” (three), etc., is added to the name to indicate the number of carboxyl groups present. For example, the compound with the formula (COOH)2 is named ethanedioic acid (also known as oxalic acid), while the compound with the formula (COOH)3 is named propanetricarboxylic acid.
It’s important to note that carboxyl groups can also be present in other types of molecules, such as esters, amides, and anhydrides. In these cases, the carboxyl group is named using the appropriate prefix (such as “carboxy-“, “acyloxy-“, or “carbonyl-“) and the “-oic acid” suffix is not used.
Case Study on Practical Organic Chemistry Carboxyl
Case Study: Synthesis of Ethyl Ethanoate
Ethyl ethanoate is an ester that is commonly used as a solvent and flavoring agent. It can be synthesized by reacting ethanoic acid with ethanol in the presence of a catalyst, such as concentrated sulfuric acid. In this case study, we will walk through the process of synthesizing ethyl ethanoate in a laboratory setting.
Materials:
- Ethanol
- Ethanoic acid
- Concentrated sulfuric acid
- Distilled water
- Separatory funnel
- Round bottom flask
- Glass condenser
- Thermometer
- Magnetic stirrer
- Ice bath
- Safety equipment (gloves, safety glasses, fume hood)
Procedure:
- Measure out 10 mL of ethanol and 10 mL of ethanoic acid using a graduated cylinder and transfer them to a round bottom flask.
- Add a few drops of concentrated sulfuric acid to the mixture and stir it using a magnetic stirrer.
- Place the flask in an ice bath to keep the temperature below 10°C.
- Add the mixture dropwise to a separatory funnel containing 20 mL of distilled water. The ester will separate as a layer on top of the water.
- Drain off the lower aqueous layer and transfer the upper organic layer to a clean beaker.
- Wash the organic layer with distilled water to remove any remaining acid.
- Dry the organic layer by adding anhydrous sodium sulfate and stirring for a few minutes.
- Filter the mixture through a funnel to remove the sodium sulfate.
- Transfer the ester to a clean, dry vial for storage and analysis.
Analysis:
The purity of the synthesized ethyl ethanoate can be analyzed using gas chromatography. The sample is injected into a gas chromatograph and the resulting chromatogram is analyzed to determine the composition of the mixture. The purity can also be determined using nuclear magnetic resonance spectroscopy.
Conclusion:
The synthesis of ethyl ethanoate is a common experiment in organic chemistry that allows students to practice techniques such as reflux, distillation, and separation using a separatory funnel. It also allows students to understand the reaction mechanism of esterification and to characterize the resulting product using analytical techniques such as gas chromatography and nuclear magnetic resonance spectroscopy. Safety precautions must be taken when working with concentrated sulfuric acid, and proper disposal of waste materials is essential.
White paper on Practical Organic Chemistry Carboxyl
Introduction:
Practical Organic Chemistry Carboxyl is an important topic in organic chemistry, which involves the study of carboxylic acids and their derivatives. Carboxylic acids are organic compounds that contain a carboxyl group (-COOH) attached to a carbon atom. They are highly versatile compounds that have a wide range of applications in many industries, including food, pharmaceuticals, and agriculture. This white paper aims to provide an overview of Practical Organic Chemistry Carboxyl, including the properties, synthesis, and applications of carboxylic acids.
Properties of Carboxylic Acids:
Carboxylic acids are polar compounds that are highly soluble in water. They have a distinctive sour taste and a pungent odor. The carboxyl group is acidic, which means that it can donate a proton (H+) to a base. Carboxylic acids also have a high boiling point and a low melting point, which makes them useful as solvents.
Synthesis of Carboxylic Acids:
Carboxylic acids can be synthesized through various methods, including:
- Oxidation of Primary Alcohols: Primary alcohols can be oxidized to carboxylic acids using an oxidizing agent, such as potassium permanganate or chromium trioxide.
- Hydrolysis of Esters: Esters can be hydrolyzed in the presence of an acid or a base to yield carboxylic acids and alcohols.
- Carbonation of Grignard Reagents: Grignard reagents can react with carbon dioxide to produce carboxylic acids.
- Oxidative Cleavage of Alkenes: Alkenes can be oxidatively cleaved using hot potassium permanganate to produce carboxylic acids.
Applications of Carboxylic Acids:
Carboxylic acids have a wide range of applications in many industries. Some of their most common uses include:
- Food Additives: Carboxylic acids are commonly used as preservatives and flavor enhancers in the food industry.
- Pharmaceuticals: Many drugs, including aspirin, penicillin, and ibuprofen, contain carboxylic acid groups.
- Polymers: Carboxylic acids are used in the production of polymers, such as nylon and polyester.
- Agriculture: Carboxylic acids are used as herbicides, fungicides, and growth regulators in agriculture.
Conclusion:
Practical Organic Chemistry Carboxyl is an important topic in organic chemistry that involves the study of carboxylic acids and their derivatives. Carboxylic acids are versatile compounds that have a wide range of applications in many industries, including food, pharmaceuticals, and agriculture. They can be synthesized through various methods and their properties make them useful as solvents and reagents. Further research on Practical Organic Chemistry Carboxyl can lead to the development of new compounds and applications in various fields.
