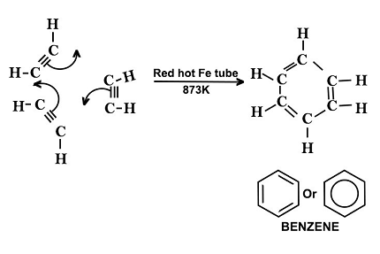
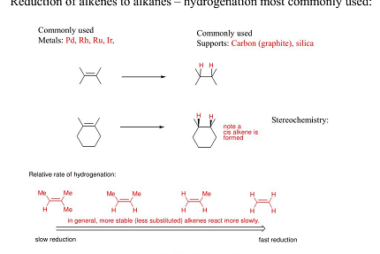
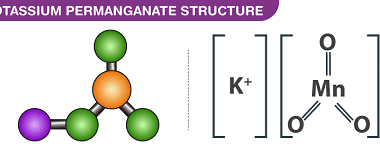
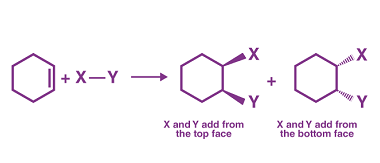
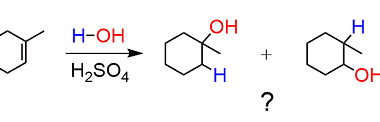
Cyclic polymerization reaction of alkynes
Cyclic polymerization of alkynes is a type of polymerization reaction in which a cyclic polymer is formed from the reaction of two or more monomers. The reaction is initiated by a suitable catalyst, typically a transition metal complex, which activates the alkyne bond to undergo polymerization. The mechanism of cyclic polymerization of alkynes involves the…