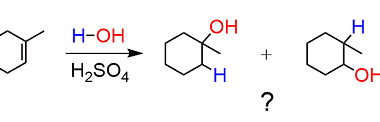
Acid catalysed hydration
Acid-catalyzed hydration is a chemical reaction in which an acid catalyst (such as sulfuric acid or phosphoric acid) is used to add water (H2O) to an unsaturated compound, typically an alkene or alkyne. The reaction produces an alcohol as the final product. The general equation for acid-catalyzed hydration of an alkene is: RCH=CH2 + H2O…