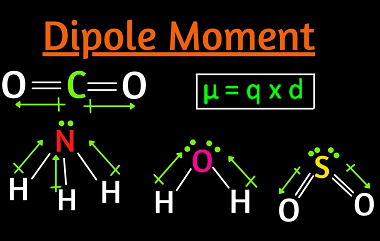
Dipole moments
A dipole moment is a measure of the separation of electrical charge within a molecule or a polar covalent bond. It occurs when there is a separation of positive and negative charges within a molecule. The magnitude of the dipole moment is represented by the product of the charge and the distance between the charges.…