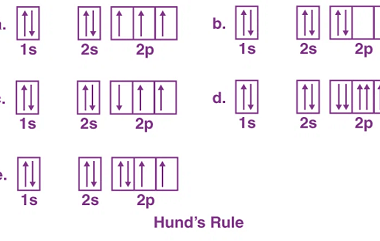
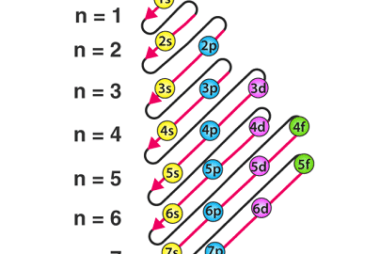
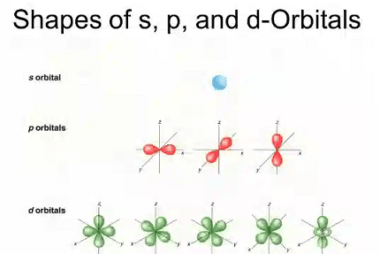
Pauli’s exclusion principle and Hund’s rule
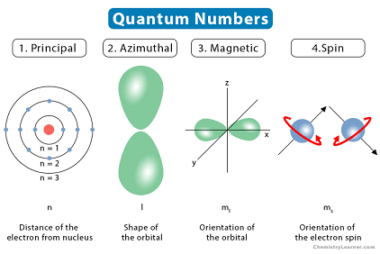
Pauli’s exclusion principle and Hund’s rule are both fundamental principles of quantum mechanics that describe the behavior of electrons in atoms. Pauli’s exclusion principle states that no two electrons in an atom can have the same set of quantum numbers. In other words, each electron in an atom must have a unique combination of values…