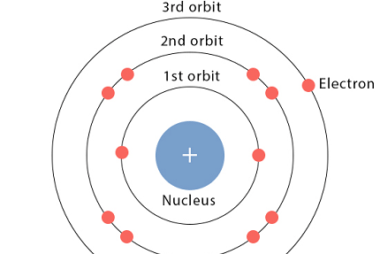
Spectrum of hydrogen atom
The spectrum of a hydrogen atom refers to the specific frequencies of electromagnetic radiation that are emitted or absorbed when an electron in a hydrogen atom changes from one energy level to another. The spectrum of hydrogen can be divided into several series, including the Lyman, Balmer, Paschen, Brackett, and Pfund series. The Lyman series…