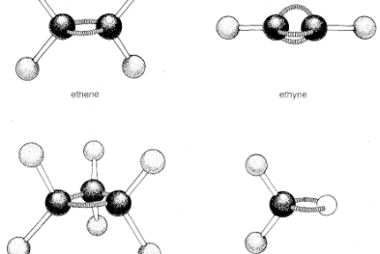
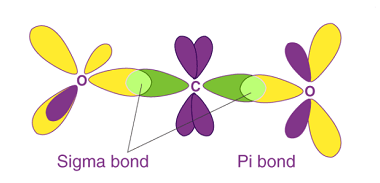
Structural and Geometrical isomerism
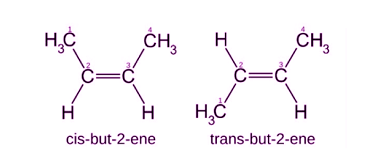
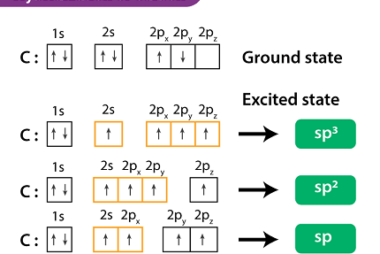
Structural isomerism and geometrical isomerism are two different types of isomerism in organic chemistry. Structural isomerism occurs when molecules have the same molecular formula but different arrangements of atoms. This can be due to differences in the bonding patterns of the atoms within the molecule. For example, pentane and 2-methylbutane have the same molecular formula…