Enzyme catalysis and Its mechanism
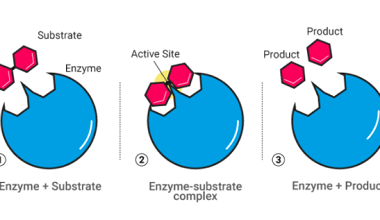
Enzymes are biological catalysts that speed up the rate of chemical reactions in living organisms. Enzymes are highly specific, meaning that they catalyze only one or a few types of chemical reactions. The mechanism by which enzymes catalyze chemical reactions is through a process called enzyme catalysis. Enzyme catalysis involves several steps: The mechanism by…