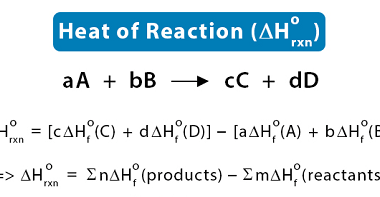Criteria of equilibrium and Spontaneity
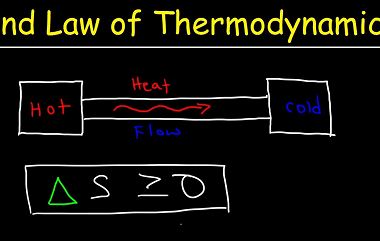
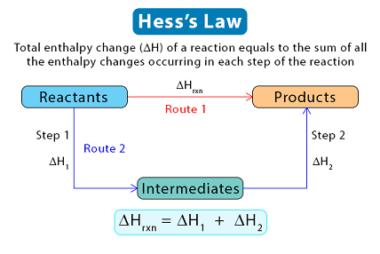
The criteria for equilibrium and spontaneity are closely related to the concept of Gibbs free energy, which is a thermodynamic function that measures the energy available in a system to do useful work. The Gibbs free energy is defined as follows: ΔG = ΔH – TΔS where ΔG is the change in Gibbs free energy,…