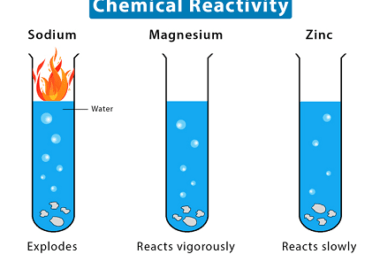
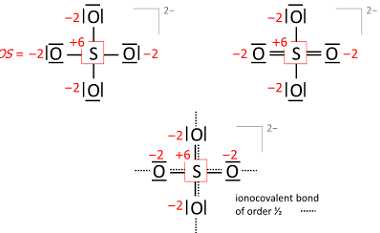
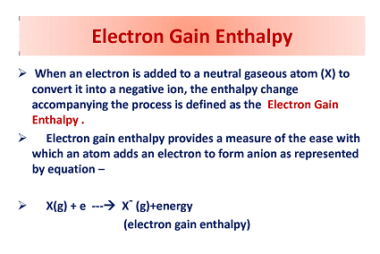
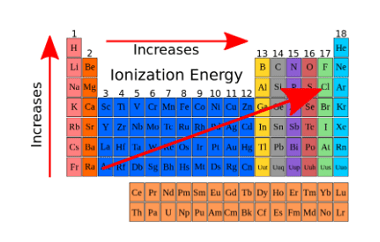
Chemical reactivity
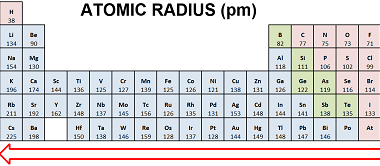
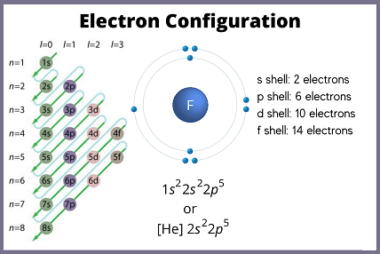
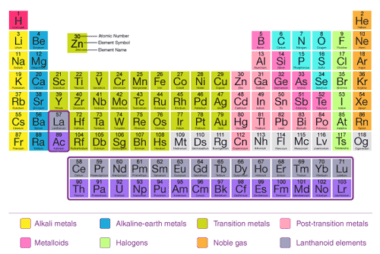
Chemical reactivity refers to the ability of a chemical substance to undergo a chemical reaction and to undergo a change in its chemical composition. It is determined by the arrangement of electrons in the atoms or molecules of a substance and their interactions with other atoms or molecules. Chemical reactivity is influenced by factors such…