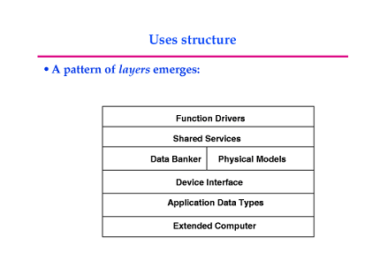
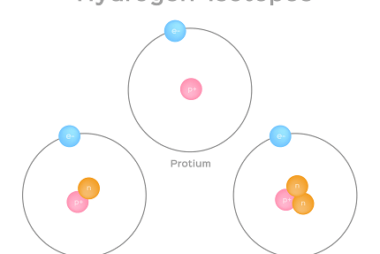
Hydrogen as a fuel
Hydrogen can be used as a fuel in a variety of ways, including as a fuel for vehicles, as a source of electricity in fuel cells, and as a feedstock for industrial processes. The appeal of hydrogen as a fuel lies in its abundance, renewability, and high energy content. When used in fuel cell vehicles,…