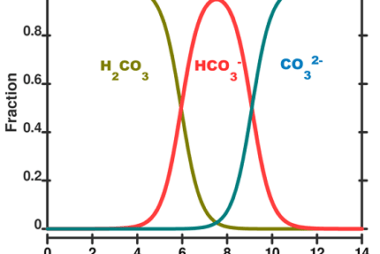
Sulphate and Sulphide
Sulphate and sulphide are two different types of chemical compounds that contain sulfur. Sulphate (or sulfate in American English) is a salt or ester of sulfuric acid. It contains the sulfate ion (SO4²⁻), which consists of one sulfur atom and four oxygen atoms. Examples of sulfate compounds include magnesium sulfate (Epsom salt), sodium sulfate (Glauber’s…