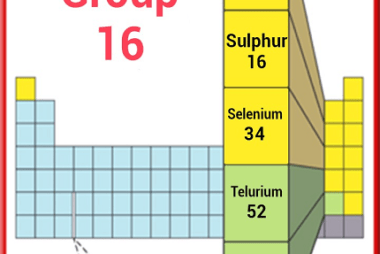
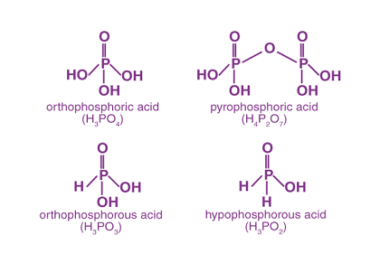
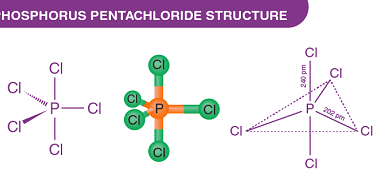
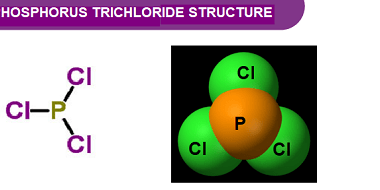
Group 16 Oxygen
Oxygen is a chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group on the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well as with other compounds. At standard temperature and pressure, two atoms of the…