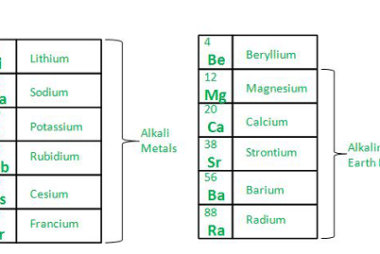
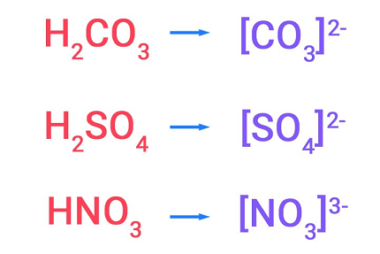
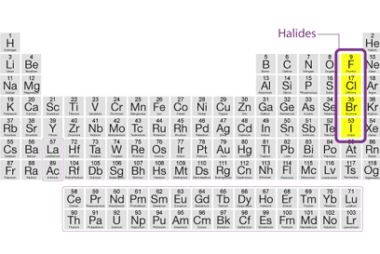
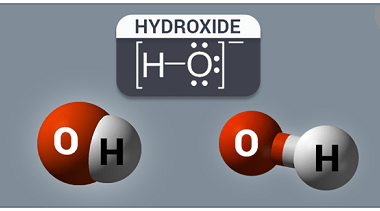
Anomalous behaviour of lithium and beryllium
Lithium and beryllium exhibit anomalous behavior due to their small size and unique electronic configurations. Lithium is the lightest metal and has a very low melting and boiling point compared to other metals. It is also the only metal that can float on water. This is due to its low density and the fact that…