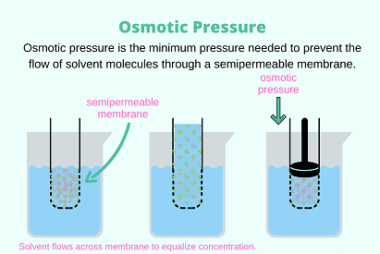
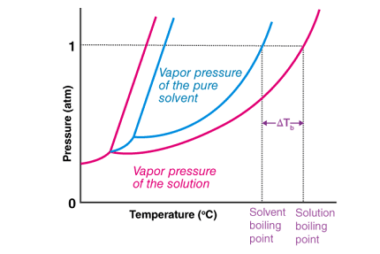
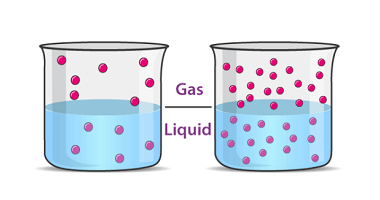
Van’t Hoff factor
The Van’t Hoff factor, also known as the Van’t Hoff factor or i-factor, is a measure of the number of particles that a solute dissociates into when it is dissolved in a solvent. The factor is named after Dutch chemist Jacobus Henricus van ‘t Hoff. The Van’t Hoff factor is defined as the ratio of…