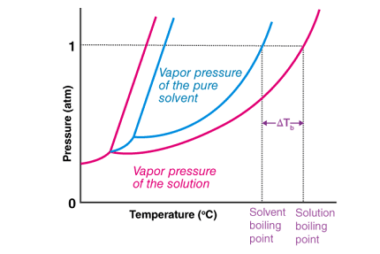
Elevation of boiling point
The elevation of boiling point refers to the increase in the boiling point of a liquid when a non-volatile solute, such as a salt or sugar, is added to it. This occurs because the presence of the solute lowers the vapor pressure of the liquid, meaning that a higher temperature is required to reach the…