Crash Course AIIMS-SYLLABUS Chemistry syllabus Catalysis of homogenous and heterogeneous
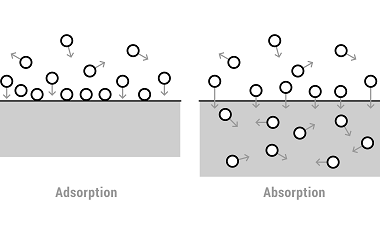
Catalysis of homogenous and heterogeneous The syllabus for the Chemistry section of the AIIMS (All India Institute of Medical Sciences) entrance exam typically covers various topics, including catalysis of homogeneous and heterogeneous reactions. Here’s a brief overview of these topics: It is important to note that the exact syllabus and depth of coverage may vary…