Crash Course AIIMS-SYLLABUS Chemistry syllabus Chemical Kinetics
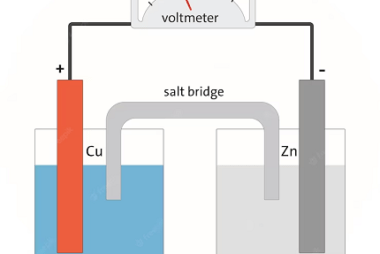
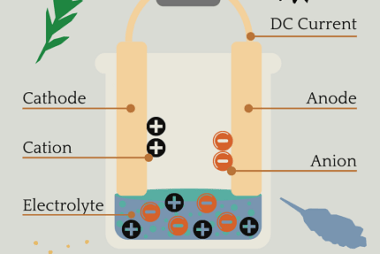
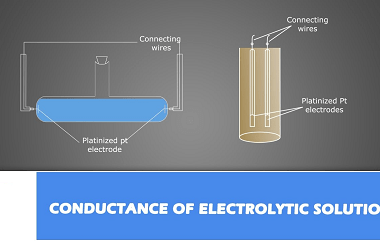
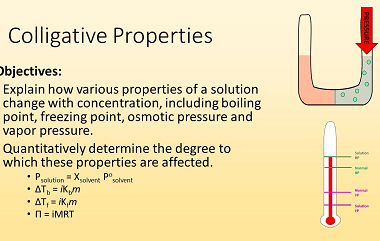
Chemical Kinetics Chemical Kinetics is an important topic in the Chemistry syllabus for AIIMS (All India Institute of Medical Sciences) entrance exam. It focuses on the study of the rates of chemical reactions and the factors that influence them. Here’s a crash course syllabus for Chemical Kinetics: It’s important to note that this crash course…