Integrated Course AIIMS-SYLLABUS Chemistry syllabus Brownian movement
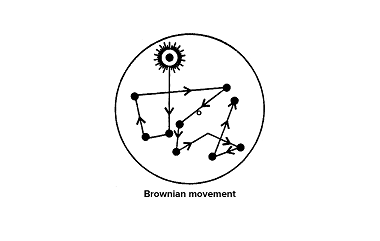
Brownian movement Brownian movement, also known as Brownian motion, refers to the random motion of microscopic particles suspended in a fluid (liquid or gas). It was first observed by the Scottish botanist Robert Brown in 1827 while studying pollen grains suspended in water. The movement is named after him. Here are some key points about…