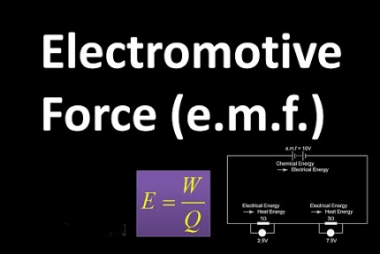
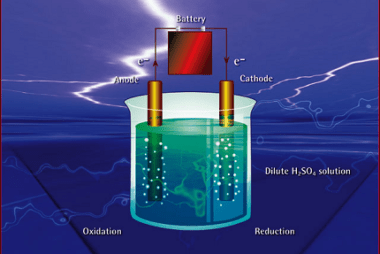
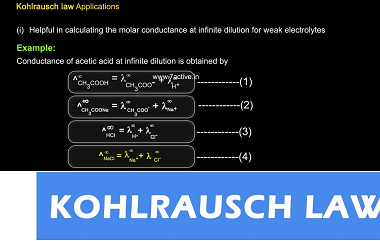
Integrated Course AIIMS-SYLLABUS Chemistry syllabus Temperature
Temperature Temperature is a fundamental physical quantity that measures the hotness or coldness of an object or a system. It is a scalar quantity and is typically measured in units such as Celsius (°C), Fahrenheit (°F), or Kelvin (K). Temperature is a macroscopic property that is related to the average kinetic energy of the particles…