Integrated Course AIIMS-SYLLABUS Chemistry syllabus Catalysis of homogenous and heterogeneous
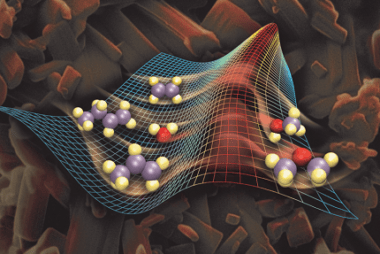
Catalysis of homogenous and heterogeneous Catalysis can occur in both homogeneous and heterogeneous systems, each with its own characteristics and mechanisms. Here’s an overview of catalysis in homogeneous and heterogeneous systems: Homogeneous Catalysis: In homogeneous catalysis, the catalyst and the reactants are present in the same phase, typically in a solution. The catalyst is usually…