Brownian movement
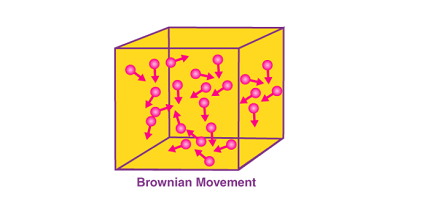
Brownian movement, also known as Brownian motion, is the random motion of microscopic particles suspended in a fluid (liquid or gas). It was first observed by the botanist Robert Brown in 1827 and later explained by Albert Einstein in 1905.
Brownian movement occurs due to the continuous collision of the fluid molecules with the suspended particles. The particles are constantly bombarded by the fast-moving fluid molecules, causing them to move in a zigzag pattern. The motion is random and unpredictable, as the particles are influenced by the cumulative effect of countless collisions from all directions.
The key factors affecting Brownian movement include the size of the particles and the temperature of the fluid. Smaller particles experience more significant Brownian motion because they are more strongly affected by collisions with fluid molecules. Additionally, higher temperatures increase the kinetic energy of the fluid molecules, leading to more vigorous particle motion.
Brownian motion has important applications in various fields, including physics, chemistry, biology, and medicine. It provides evidence for the existence of atoms and molecules, as the random motion of particles is attributed to the individual collisions with these tiny particles. Brownian motion is also utilized in techniques such as Brownian dynamics simulations and particle tracking to study and analyze systems at the microscopic level.
The AIIMS Chemistry syllabus covers various topics, including Brownian movement. Brownian movement refers to the random motion of microscopic particles suspended in a fluid due to collisions with the fluid molecules. It is caused by the thermal energy of the fluid molecules. The key concepts related to Brownian movement that may be covered in the syllabus include:
- Explanation of Brownian motion: Understanding the random motion of particles and the factors affecting it, such as particle size and temperature.
- Kinetic theory of gases: Introduction to the kinetic theory, which explains the behavior of gases in terms of the motion of their constituent particles, including Brownian motion.
- Brownian motion and diffusion: Exploring the relationship between Brownian motion and the process of diffusion, where particles move from an area of higher concentration to lower concentration.
- Einstein’s explanation: Briefly studying Albert Einstein’s explanation of Brownian motion, which involved mathematical modeling and theoretical analysis.
It’s important to note that the exact depth and extent of the topic within the AIIMS syllabus may vary, so referring to the official syllabus or course materials would provide a more comprehensive understanding of the specific content to be covered.
What is Required Chemistry syllabus Brownian movement
The specific details of the required chemistry syllabus regarding Brownian movement can vary depending on the educational institution or curriculum. However, in a general chemistry syllabus, the topic of Brownian movement may be covered as part of the following concepts:
- States of matter: Introduction to the three states of matter (solid, liquid, gas) and the behavior of particles in each state. Brownian motion is often discussed in the context of particles in a fluid (liquid or gas).
- Kinetic theory of gases: Overview of the kinetic theory, which explains the behavior of gases based on the motion of their particles. Brownian motion can be linked to the kinetic theory, highlighting the random movement of particles in a gas.
- Diffusion: Understanding the process of diffusion, where particles move from an area of higher concentration to lower concentration. Brownian motion plays a role in diffusion as particles undergo random motion and collisions, leading to their dispersal.
- Colloids: Discussion of colloidal systems, where small particles are dispersed in a continuous medium (liquid or gas). Brownian motion is significant in colloidal systems as it contributes to the stability of colloids by preventing the particles from settling.
These are some general areas where Brownian movement might be included in a chemistry syllabus. It’s recommended to refer to the specific syllabus or course materials provided by your educational institution for a more detailed and accurate understanding of the topics covered.
When is Required Chemistry syllabus Brownian movement
The inclusion of the topic of Brownian movement in the required chemistry syllabus can vary depending on the educational institution or curriculum. In general, Brownian movement is often covered in introductory or foundational courses in chemistry, particularly those that focus on the behavior of matter and the properties of particles.
In secondary education, Brownian movement may be introduced in high school chemistry courses as part of topics related to states of matter, kinetic theory, and diffusion. It may be discussed in the context of understanding the behavior of particles in gases and liquids.
At the undergraduate level, Brownian movement can be included in introductory or general chemistry courses. It may also be covered in more advanced courses such as physical chemistry or materials science, where a deeper understanding of particle behavior and the properties of colloids is explored.
In specialized fields of study such as chemical engineering or biophysics, the topic of Brownian movement may be covered in more detail, with applications to specific areas such as nanoparticle research, drug delivery systems, or the study of biological macromolecules.
It’s important to consult the specific chemistry syllabus or course outline provided by your educational institution to determine the exact timing and extent of the topic’s coverage.
Where is Required Chemistry syllabus Brownian movement
The inclusion of the topic of Brownian movement in the required chemistry syllabus can vary depending on the educational institution or curriculum. Typically, it can be found within the broader topics of physical chemistry, colloidal chemistry, or introductory chemistry courses that cover states of matter, kinetic theory, and particle behavior. Here are a few common places where Brownian movement may be included in the chemistry syllabus:
- Introduction to Physical Chemistry: Brownian motion is often introduced as part of the discussion on the kinetic theory of gases and the behavior of particles. This section may cover concepts such as ideal gas behavior, molecular motion, and the relationship between temperature and particle motion.
- Colloidal Chemistry: Brownian motion is particularly relevant in the study of colloids, which are systems where small particles are dispersed in a continuous medium. The syllabus may include a section on colloidal stability, where Brownian motion is discussed as a mechanism that prevents particles from settling.
- Introduction to States of Matter: In courses that cover the different states of matter (solid, liquid, gas), Brownian motion may be introduced as part of the discussion on particle behavior in fluids. This section may explore how Brownian motion contributes to the diffusion of particles in liquids or the movement of gas molecules.
It’s important to note that the specific location of Brownian movement within the syllabus can vary, and some institutions or curricula may provide a more specialized or in-depth treatment of the topic. Therefore, it is advisable to consult the chemistry syllabus or course outline provided by your educational institution to determine the exact placement of Brownian movement within the curriculum.
How is Required Chemistry syllabus Brownian movement
The specific treatment of Brownian movement in the required chemistry syllabus can vary depending on the educational institution or curriculum. However, in general, the syllabus may cover Brownian movement in the following manner:
- Definition and Historical Background: The syllabus may introduce the concept of Brownian movement and provide a historical context, highlighting the observations made by Robert Brown and the subsequent explanation by Albert Einstein.
- Explanation of Brownian Motion: The syllabus may delve into the underlying principles of Brownian motion, emphasizing the random motion of microscopic particles suspended in a fluid. It may cover the role of collisions between particles and fluid molecules, as well as the influence of temperature and particle size on the intensity of Brownian motion.
- Kinetic Theory of Gases: Brownian movement is often discussed within the broader context of the kinetic theory of gases. The syllabus may cover the basic principles of the kinetic theory, including the concept of particles in motion and the relationship between temperature, pressure, and particle behavior.
- Diffusion and Brownian Motion: The syllabus may explore the connection between Brownian motion and diffusion, emphasizing how the random movement of particles contributes to the process of diffusion. It may discuss how Brownian motion facilitates the mixing and spreading of particles in a fluid.
- Applications and Significance: The syllabus may highlight the significance of Brownian motion in various scientific and technological areas. It may cover applications in fields such as physics, chemistry, biology, and medicine, including topics such as nanoparticle research, drug delivery systems, and the study of colloidal systems.
It’s important to note that the depth and extent of the topic’s coverage can vary. Therefore, consulting the specific chemistry syllabus or course materials provided by your educational institution would provide a more accurate and comprehensive understanding of how Brownian movement is addressed in your particular curriculum.
Nomenclature of Chemistry syllabus Brownian movement
The specific nomenclature used for the topic of Brownian movement in the chemistry syllabus can vary depending on the educational institution or curriculum. However, here are some common terms and keywords that may be associated with the topic:
- Brownian motion
- Brownian movement
- Kinetic theory of gases
- Diffusion and Brownian motion
- Particle motion in fluids
- Random motion of particles
- Thermal motion and collisions
- Colloidal stability and Brownian motion
- Particle behavior in liquids and gases
- Relationship between temperature and particle motion
These terms and keywords are typically used to describe and categorize the topic of Brownian movement within the chemistry syllabus. It’s important to refer to the specific syllabus or course materials provided by your educational institution to understand the exact nomenclature and terminology used in your curriculum.
Case Study on Chemistry syllabus Brownian movement
Title: Investigating Brownian Movement: A Case Study
Introduction: Brownian movement, also known as Brownian motion, is a fascinating phenomenon observed in nature. In this case study, we will explore a real-world application of Brownian movement and examine how it is utilized in the field of nanoparticle research.
Background: Nanoparticles are tiny particles with dimensions ranging from 1 to 100 nanometers. Due to their small size, nanoparticles exhibit unique properties that differ from those of bulk materials. Brownian movement plays a crucial role in nanoparticle research as it affects their dispersion, stability, and interaction with the surrounding environment.
Objective: The objective of this case study is to investigate the influence of Brownian movement on nanoparticle behavior and understand its implications for applications in various fields.
Methodology:
- Selection of Nanoparticles: Choose a specific type of nanoparticle for the study, such as gold nanoparticles or quantum dots. These nanoparticles can be synthesized or obtained from commercial sources.
- Particle Tracking: Utilize advanced microscopy techniques, such as video microscopy or laser tracking, to observe and track the motion of individual nanoparticles in a liquid medium. Record videos or capture images of the nanoparticles’ motion over time.
- Data Analysis: Analyze the recorded data to determine the trajectories and velocities of the nanoparticles. Use software tools or algorithms to track and quantify the Brownian motion exhibited by the particles.
- Brownian Motion Parameters: Calculate parameters such as mean square displacement, diffusion coefficient, and root mean square velocity to characterize the Brownian motion of the nanoparticles. Compare the obtained values with theoretical predictions.
- Effects of Brownian Motion: Investigate how Brownian movement affects the dispersion and stability of nanoparticles in the liquid medium. Examine the role of Brownian motion in particle-particle interactions, aggregation, and sedimentation.
- Applications: Discuss potential applications of nanoparticle research that rely on Brownian motion, such as drug delivery systems, environmental remediation, catalysis, or biomedical imaging. Highlight how an understanding of Brownian movement is essential for designing effective nanoparticle-based technologies.
Conclusion: Summarize the findings of the case study, emphasizing the significance of Brownian movement in nanoparticle research. Reflect on the practical implications and potential future developments in the field. Highlight the relevance of understanding Brownian motion for various applications and the importance of continued research in this area.
This case study demonstrates how investigating Brownian movement in the context of nanoparticle research provides insights into particle behavior and paves the way for advancements in nanotechnology and related fields.
White paper on Chemistry syllabus Brownian movement
Title: White Paper on Brownian Movement: Understanding the Fundamental Principles and Applications
Abstract:
This white paper provides a comprehensive overview of Brownian movement, a fundamental concept in physics and chemistry. We explore the underlying principles, historical background, mathematical modeling, and real-world applications of Brownian motion. The paper aims to enhance the understanding of this phenomenon and its significance in various scientific disciplines and practical applications.
Introduction:
1.1 Historical Background: Briefly discuss the observations made by Robert Brown and the subsequent explanation by Albert Einstein.
1.2 Definition and Nature of Brownian Movement: Define Brownian movement as the random motion of microscopic particles suspended in a fluid due to collisions with fluid molecules.
Mechanisms and Principles:
2.1 Kinetic Theory of Gases: Explain how Brownian motion is related to the kinetic theory and the behavior of gas molecules.
2.2 Diffusion: Discuss the connection between Brownian motion and the process of diffusion, highlighting the role of random particle motion in spreading and mixing.
Mathematical Modeling:
3.1 Einstein’s Work: Describe Albert Einstein’s contribution to the understanding of Brownian motion, including his mathematical model and the relationship between particle size, temperature, and diffusion.
3.2 Brownian Dynamics Simulation: Introduce computational methods such as Brownian dynamics simulations that use numerical techniques to simulate and analyze Brownian motion.
Applications:
4.1 Particle Characterization: Discuss how Brownian motion is used for particle size determination and the study of particle properties.
4.2 Colloidal Systems: Explore the role of Brownian motion in the stability and behavior of colloidal systems, including colloidal suspensions and emulsions.
4.3 Nanoparticle Research: Highlight the importance of Brownian motion in nanoparticle synthesis, dispersion, and applications such as drug delivery systems and nanoscale imaging.
4.4 Biological Systems: Explain how Brownian motion is relevant in biological systems, including the movement of molecules within cells and the behavior of biomolecules.
Experimental Techniques:
5.1 Particle Tracking: Describe experimental methods like video microscopy or laser tracking that allow for the observation and analysis of Brownian motion.
5.2 Single-Particle Tracking: Discuss advanced techniques that enable the tracking and analysis of individual particles, providing insights into their trajectories, diffusion coefficients, and interactions.
Future Directions and Challenges:
6.1 Advanced Analysis Techniques: Highlight emerging techniques and technologies for studying Brownian motion, such as super-resolution microscopy and machine learning-based analysis.
6.2 Complex Systems: Discuss the challenges and opportunities in understanding Brownian motion in complex systems, including non-spherical particles, confined environments, and crowded media.
Conclusion:
Summarize the key points discussed in the white paper, emphasizing the fundamental nature and broad applications of Brownian movement. Highlight the importance of continued research and technological advancements to unlock the full potential of this phenomenon.
By providing a comprehensive understanding of Brownian motion, this white paper aims to serve as a valuable resource for researchers, scientists, and students across various scientific disciplines. It underscores the significance of Brownian movement in both fundamental scientific understanding and practical applications in diverse fields.
