Chemisorption
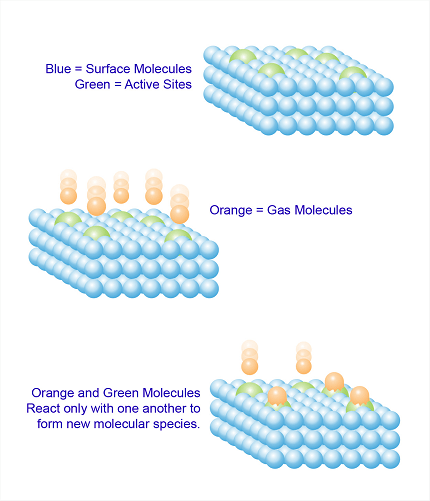
Chemisorption is a process of adsorption in which a chemical reaction occurs between the adsorbate (molecules or atoms being adsorbed) and the surface of the adsorbent (the material on which adsorption takes place). It involves the formation of chemical bonds between the adsorbate and adsorbent, resulting in the creation of a new compound or species on the surface.
In chemisorption, the adsorbate molecules are strongly bound to the adsorbent surface through covalent or ionic bonds. This type of adsorption is usually specific, meaning that it is selective towards certain types of adsorbates or adsorbents.
Some key features of chemisorption are:
- Bond formation: Chemisorption involves the formation of chemical bonds between the adsorbate and adsorbent, leading to the adsorbed species becoming a part of the adsorbent surface.
- Energy changes: Chemisorption is typically characterized by a high energy of adsorption due to the formation of strong chemical bonds.
- Activation energy: Chemisorption often requires a certain minimum energy (activation energy) for the reaction between the adsorbate and adsorbent to occur.
- Specificity: Chemisorption is more specific than physisorption (adsorption based on weak intermolecular forces) and is influenced by factors such as the nature of the adsorbate and adsorbent, as well as temperature and pressure.
Chemisorption has various applications, particularly in catalysis, where the adsorbed species act as active sites for chemical reactions. It is also important in fields such as gas purification, surface science, and heterogeneous reactions.
The syllabus for the advanced course at AIIMS includes the topic of chemisorption in Chemistry. Chemisorption refers to the process of adsorption where there is a chemical reaction between the adsorbate (molecules or atoms being adsorbed) and the surface of the adsorbent (the material on which adsorption takes place). It involves the formation of chemical bonds between the adsorbate and adsorbent, resulting in the formation of a new compound or species on the surface.
In the context of the AIIMS syllabus, the topic of chemisorption may cover the following aspects:
- Adsorption isotherms: Describing the relationship between the amount of adsorbate and the pressure or concentration at a constant temperature.
- Factors influencing chemisorption: The effect of temperature, pressure, and surface characteristics on the rate and extent of chemisorption.
- Mechanism of chemisorption: Understanding the steps involved in the chemisorption process, such as activation of adsorbate molecules, migration and diffusion on the surface, and formation of chemical bonds.
- Types of chemisorption: Differentiating between specific and non-specific chemisorption based on the strength and selectivity of the chemical bonds formed.
- Applications of chemisorption: Exploring the practical uses of chemisorption in various fields, such as catalysis, gas purification, and heterogeneous reactions.
It is important to note that the specific details and depth of coverage for the topic of chemisorption in the AIIMS syllabus may vary depending on the course level and curriculum.
What is Required Chemistry syllabus Chemisorption
The specific details of the chemistry syllabus on chemisorption may vary depending on the educational institution and level of study. However, here are some common topics that are typically covered in the chemistry syllabus related to chemisorption:
- Adsorption: Introduction to adsorption and its classification into physisorption and chemisorption. Understanding the factors influencing adsorption, such as temperature, pressure, and surface characteristics.
- Chemisorption: Definition of chemisorption and its differences from physisorption. Discussion of the characteristics of chemisorption, including the formation of chemical bonds and the specificity of the process.
- Adsorption isotherms: Study of the relationship between the amount of adsorbate and the pressure or concentration at a constant temperature. Understanding different types of adsorption isotherms, including Langmuir and Freundlich isotherms.
- Activation energy: Explanation of the concept of activation energy in chemisorption and its significance in determining the rate of chemisorption reactions.
- Factors influencing chemisorption: Detailed analysis of the factors affecting chemisorption, such as temperature, pressure, nature of adsorbate and adsorbent, and surface area.
- Mechanism of chemisorption: Discussion of the steps involved in chemisorption, including adsorbate activation, migration and diffusion on the surface, and the formation of chemical bonds.
- Catalysis: Introduction to catalysis and its relationship to chemisorption. Understanding how chemisorption plays a crucial role in catalytic reactions and the concept of active sites.
- Applications of chemisorption: Exploring the practical applications of chemisorption in various fields, such as catalysis, gas purification, heterogeneous reactions, and surface science.
It is important to refer to the specific syllabus provided by your educational institution or course instructor to ensure you have the complete and accurate details of the chemistry syllabus on chemisorption.
When is Required Chemistry syllabus Chemisorption
The topic of chemisorption is typically covered in the chemistry syllabus at various educational levels, depending on the curriculum and depth of study. Here are some common instances when the chemistry syllabus may include chemisorption:
- Undergraduate level: Chemisorption is often included as part of the physical chemistry or surface chemistry courses in undergraduate programs. It is commonly taught in chemistry or chemical engineering degrees during the second or third year of study.
- Postgraduate level: Chemisorption is an important topic in advanced courses and research programs focusing on surface chemistry, catalysis, materials science, or related disciplines at the postgraduate level.
- Entrance examinations: Chemisorption may be included as part of the syllabus for entrance examinations for higher education programs in chemistry or chemical sciences, such as national level entrance exams for postgraduate admissions.
- Specialized courses: In specialized programs or courses with a specific focus on surface science, adsorption, or catalysis, chemisorption may be given more emphasis and studied in greater detail.
It’s worth noting that the specific timing and inclusion of chemisorption in the chemistry syllabus can vary between educational institutions and regions. It is recommended to refer to the syllabus provided by your institution or consult with your course instructor for accurate information on when chemisorption is covered in your particular curriculum.
Where is Required Chemistry syllabus Chemisorption
The chemistry syllabus that includes the topic of chemisorption can be found in various educational settings, such as:
- Universities and Colleges: Chemistry departments or faculties in universities and colleges typically have a structured syllabus that outlines the topics covered in different courses. Chemisorption may be included in courses such as physical chemistry, surface chemistry, catalysis, or materials science.
- Secondary Education: In some educational systems, chemistry syllabi at the secondary education level (high school or equivalent) may cover basic concepts of adsorption, including an introduction to chemisorption. However, the depth of coverage may be more limited compared to higher education levels.
- Entrance Examination Syllabi: Chemisorption may be included as part of the syllabus for entrance examinations for admission to undergraduate or postgraduate programs in chemistry or related fields. These entrance exam syllabi are often provided by the organizing bodies responsible for the entrance exams.
It is important to note that the specific location of the chemisorption topic within the chemistry syllabus can vary depending on the institution and the specific course or program. To obtain the most accurate and up-to-date information on the inclusion and placement of chemisorption in the syllabus, it is recommended to refer to the syllabus provided by your educational institution or consult with your course instructor or department.
How is Required Chemistry syllabus Chemisorption
The chemistry syllabus on chemisorption typically includes theoretical concepts, practical applications, and experimental techniques related to this topic. Here is a general overview of how the required chemistry syllabus on chemisorption may be structured:
- Introduction to Adsorption: The syllabus may begin with an introduction to adsorption as a phenomenon, discussing the difference between physical adsorption (physisorption) and chemical adsorption (chemisorption). The importance of chemisorption in various chemical processes and applications can also be emphasized.
- Adsorption Isotherms: The syllabus may cover different types of adsorption isotherms, including the Langmuir isotherm and the Freundlich isotherm. Students may learn how to interpret and analyze adsorption isotherm data to understand the nature of chemisorption.
- Factors Affecting Chemisorption: This section of the syllabus may delve into the factors that influence the rate and extent of chemisorption. Topics covered may include temperature, pressure, surface area, nature of adsorbate and adsorbent, and the role of catalysts in promoting chemisorption.
- Mechanism and Kinetics of Chemisorption: Students may study the stepwise mechanism of chemisorption, including adsorbate activation, adsorption and diffusion on the surface, and the formation of chemical bonds. Kinetic aspects of chemisorption, such as activation energy and rate equations, may also be covered.
- Experimental Techniques: The syllabus may include an overview of experimental techniques used to study chemisorption. This may include techniques such as adsorption isotherm measurements, surface area determination, temperature-programmed desorption (TPD), and techniques for characterizing the adsorbent surface (e.g., X-ray photoelectron spectroscopy, infrared spectroscopy).
- Applications of Chemisorption: The syllabus may explore the practical applications of chemisorption in various fields, such as catalysis, gas purification, adsorption-based separations, and surface science. Students may learn about specific examples and case studies highlighting the significance of chemisorption in these applications.
It is important to note that the specific content and depth of coverage within the chemistry syllabus on chemisorption may vary depending on the educational institution, level of study, and the duration of the course. It is recommended to refer to the syllabus provided by your educational institution for a detailed outline of the required topics and subtopics in the chemistry syllabus on chemisorption.
Case Study on Chemistry syllabus Chemisorption
Case Study: Chemisorption in Catalysis
Chemisorption plays a crucial role in catalytic processes, where it helps facilitate chemical reactions by providing active sites on the catalyst surface. Let’s consider a case study that demonstrates the significance of chemisorption in catalysis.
Case Study: Hydrogenation of Ethylene
In the production of various chemicals, such as ethylene glycol or polyethylene, the hydrogenation of ethylene is an essential step. This process involves the addition of hydrogen gas (H2) to ethylene (C2H4) to form ethane (C2H6). Chemisorption is vital in enabling this catalytic reaction to occur efficiently.
- Catalyst Selection: A suitable catalyst for this reaction is often a metal, such as platinum (Pt), palladium (Pd), or nickel (Ni), supported on a solid material like alumina (Al2O3). The choice of catalyst depends on factors such as activity, selectivity, and cost.
- Chemisorption of Hydrogen: The first step in the hydrogenation process is the adsorption of hydrogen on the catalyst surface. Hydrogen molecules chemisorb onto the active sites of the catalyst by breaking the H-H bond and forming chemical bonds with the metal surface.
- Chemisorption of Ethylene: Ethylene molecules, present in the gas phase, chemisorb onto the catalyst surface as well. The π-bonds in ethylene break, and the resulting ethylidyne (C2H3) intermediates form chemical bonds with the catalyst.
- Surface Reaction: The chemisorbed ethylidyne species and the chemisorbed hydrogen atoms react on the catalyst surface, facilitating the addition of hydrogen to the ethylene molecule. This results in the formation of ethane.
- Desorption: The final step involves the desorption of the ethane product from the catalyst surface. The weakened chemical bonds between the ethane molecule and the catalyst allow the product to be released.
Chemisorption is critical for the catalytic hydrogenation of ethylene because it provides an active site for the reaction to take place. The chemisorbed species, such as hydrogen and ethylidyne, act as intermediates that enable the formation of new chemical bonds and the conversion of reactants into products.
The efficiency and selectivity of the hydrogenation process depend on the strength and stability of the chemisorbed species on the catalyst surface. Factors such as temperature, pressure, and catalyst composition can influence the extent of chemisorption and, consequently, the overall catalytic activity.
Understanding the chemisorption process in catalysis allows scientists and engineers to optimize catalyst design, control reaction conditions, and improve the efficiency of industrial processes. It highlights the importance of surface science and the role of chemisorption in driving chemical transformations on solid catalysts.
White paper on Chemistry syllabus Chemisorption
Title: Understanding Chemisorption: Mechanisms, Applications, and Future Perspectives
Abstract: Chemisorption, a fundamental process in surface science and catalysis, involves the formation of chemical bonds between adsorbate molecules and the surface of an adsorbent material. This white paper provides a comprehensive overview of chemisorption, covering its mechanisms, applications in various industries, and potential future directions for research and development. By understanding the intricacies of chemisorption, scientists and engineers can harness its power to drive advancements in catalysis, materials science, and surface chemistry.
- Introduction
- Definition and distinction between chemisorption and physisorption.
- Significance of chemisorption in catalysis, gas separations, and surface science.
- Mechanisms of Chemisorption
- Adsorbate activation and dissociation on the surface.
- Surface diffusion and migration of adsorbate species.
- Formation of chemical bonds between adsorbate and adsorbent.
- Factors Influencing Chemisorption
- Temperature, pressure, and concentration effects.
- Role of surface area, morphology, and composition.
- Selectivity and specificity of chemisorption reactions.
- Experimental Techniques for Studying Chemisorption
- Adsorption isotherm measurements and interpretation.
- Surface analysis techniques (XPS, IR spectroscopy, TPD, etc.).
- In situ and operando spectroscopic techniques.
- Applications of Chemisorption
- Catalysis: Hydrogenation, oxidation, and selective transformations.
- Gas purification and separation processes.
- Surface modification and functionalization.
- Environmental applications and pollution control.
- Emerging Trends and Future Perspectives
- Design of novel catalysts for specific chemisorption processes.
- Advances in computational modeling and simulation of chemisorption.
- Integration of chemisorption with other surface phenomena (e.g., electrochemistry).
- Expanding applications in renewable energy, pharmaceuticals, and nanotechnology.
- Challenges and Opportunities
- Overcoming limitations in catalyst stability and selectivity.
- Enhancing understanding of complex chemisorption processes.
- Developing sustainable and eco-friendly chemisorption processes.
- Conclusion
- Recap of key findings and the importance of chemisorption in various fields.
- Potential future directions for research and technological advancements.
This white paper aims to provide a comprehensive and up-to-date understanding of chemisorption, highlighting its mechanisms, applications, and future prospects. By embracing the potential of chemisorption, researchers and industrial practitioners can advance the development of efficient and sustainable processes across multiple disciplines.
