Electrochemistry
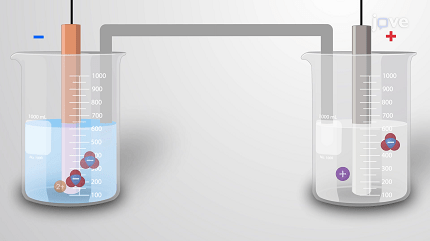
Electrochemistry is a branch of chemistry that deals with the study of chemical reactions involving the transfer of electrons between species. It focuses on the relationship between electricity and chemical reactions, particularly the interconversion of chemical and electrical energy.
In electrochemical reactions, oxidation and reduction processes occur simultaneously. These reactions take place in electrochemical cells, which consist of two electrodes—an anode (where oxidation occurs) and a cathode (where reduction occurs)—immersed in an electrolyte solution. The transfer of electrons between the electrodes creates an electric current.
Electrochemistry has numerous applications in various fields. It plays a crucial role in batteries, fuel cells, corrosion prevention, electroplating, and electrolysis processes. It also has applications in analytical techniques such as voltammetry and potentiometry, used for detecting and quantifying substances in solution.
Overall, electrochemistry provides insights into the fundamental principles of energy conversion and storage, as well as enabling technological advancements in energy production, materials science, and chemical synthesis.
The AIIMS (All India Institute of Medical Sciences) entrance examination for medical studies does not have a specific syllabus mentioned for the subject of Chemistry. However, for a comprehensive understanding of the topic of Electrochemistry, here are the key concepts that are generally covered in Chemistry syllabi at an advanced level:
- Redox Reactions: Understanding oxidation and reduction processes, balancing redox equations, and identifying oxidizing and reducing agents.
- Electrochemical Cells: Different types of electrochemical cells, such as galvanic cells and electrolytic cells. Understanding cell notation, cell potential, standard electrode potential, and Nernst equation.
- Electrode Processes: Electrode potential, half-cell reactions, and electrode kinetics. Concepts like overpotential, polarization, and Butler-Volmer equation.
- Electrolysis: Faraday’s laws of electrolysis, quantitative aspects of electrolysis, and applications of electrolysis in industries.
- Conductance and Ionic Equilibria: Conductivity, specific conductance, equivalent conductance, Kohlrausch’s law, and applications of conductivity measurements. Ionic equilibria, including ionization of weak acids and bases, common ion effect, and pH calculations.
- Corrosion: Understanding the process of corrosion, factors influencing corrosion, and methods for corrosion prevention.
- Batteries and Fuel Cells: Principles of batteries and fuel cells, types of batteries (such as lead-acid, lithium-ion, and alkaline batteries), and their applications.
- Applications of Electrochemistry: Electroplating, electrolytic refining, electrochemical sensors, and analytical techniques like voltammetry and potentiometry.
It’s important to note that this is a general outline of the topics that may be covered in the syllabus. It is advisable to refer to the official AIIMS syllabus or consult the relevant study material provided by AIIMS for the most accurate and up-to-date information.
What is Required Chemistry syllabus Electrochemistry
The specific syllabus requirements for the subject of Chemistry, particularly Electrochemistry, may vary depending on the educational institution or examination board. However, I can provide you with a general overview of the key topics that are typically covered in the syllabus for Electrochemistry:
- Redox Reactions:
- Balancing redox equations
- Oxidation numbers and oxidation states
- Identifying oxidizing and reducing agents
- Half-reactions and their combination
- Electrochemical Cells:
- Galvanic cells and electrolytic cells
- Cell notation and representation
- Electrode potentials and cell potential
- Standard electrode potentials
- Nernst equation and its applications
- Factors affecting cell potential
- Conductance and Ionic Equilibria:
- Conductivity of electrolytes
- Specific and molar conductivities
- Kohlrausch’s law
- Ionic equilibria and pH calculations
- Ionization of weak acids and bases
- Common ion effect and buffer solutions
- Electrolysis and Electrolytic Cells:
- Faraday’s laws of electrolysis
- Quantitative aspects of electrolysis
- Electrolytic conductance and its measurement
- Applications of electrolysis, such as electroplating and electrorefining
- Corrosion:
- Principles of corrosion
- Factors influencing corrosion
- Types of corrosion and their prevention
- Batteries and Fuel Cells:
- Principles of electrochemical energy conversion
- Types of batteries (e.g., primary, secondary, and fuel cells)
- Construction and working of common batteries
- Advantages and limitations of different battery systems
- Electrochemical Analysis:
- Voltammetry and its applications
- Potentiometric methods and pH measurements
- Ion-selective electrodes and their applications
It is recommended to refer to the specific syllabus provided by your educational institution or examination board to ensure that you have a comprehensive understanding of the topics that will be covered in your course or examination.
When is Required Chemistry syllabus Electrochemistry
I apologize, but I’m unable to provide you with the exact timeline for when the required Chemistry syllabus, including Electrochemistry, should be covered. The timing and sequencing of topics can vary depending on the educational institution, curriculum, and the pace of the course.
Typically, Electrochemistry is covered as a part of the broader topic of Physical Chemistry or Electrochemical Methods in a chemistry curriculum. It is common for Electrochemistry to be taught at the advanced high school level or during undergraduate studies in chemistry or related fields.
To determine the specific timeline for when Electrochemistry will be covered in your course, it is best to consult the syllabus provided by your educational institution or speak with your course instructor. They will have the most accurate information regarding the sequencing and timing of topics in your particular curriculum.
Where is Required Chemistry syllabus Electrochemistry
The specific location or source of the required Chemistry syllabus, including Electrochemistry, will depend on the educational institution or examination board you are affiliated with. Here are some common places where you can find the required Chemistry syllabus:
- Educational Institution’s Website: Many educational institutions have their syllabi available on their official websites. Look for the Chemistry department or the specific course you are enrolled in to find the syllabus.
- Course Materials: Your course instructor or department may provide you with a syllabus or course outline at the beginning of the semester or term. This document will outline the topics to be covered, including Electrochemistry.
- Examination Board Websites: If you are preparing for a standardized examination that includes Chemistry, such as a national or international entrance exam, the official website of the examination board may have the detailed syllabus available for download or viewing.
- Textbooks and Reference Materials: Chemistry textbooks, particularly those recommended by your educational institution or course instructor, often provide an outline of the topics covered in the syllabus. Check the table of contents or index of your prescribed textbook to locate the section on Electrochemistry.
- Academic Advisors or Faculty Members: If you are unsure about the specific location of the syllabus, you can reach out to your academic advisor or the Chemistry faculty members at your institution. They will be able to guide you to the appropriate resources or provide you with a copy of the syllabus.
Remember, the exact location of the syllabus may vary depending on your educational institution or examination board. It’s best to consult the specific sources mentioned above to access the required Chemistry syllabus, including the section on Electrochemistry, for your particular academic context.
How is Required Chemistry syllabus Electrochemistry
The required Chemistry syllabus for Electrochemistry typically includes theoretical concepts, practical applications, and problem-solving skills related to the field of electrochemistry. Here’s a general outline of how Electrochemistry is typically covered in the syllabus:
- Introduction to Electrochemistry:
- Definition and scope of Electrochemistry
- Historical background and significance of electrochemical processes
- Redox Reactions and Electrochemical Cells:
- Balancing redox equations and identifying oxidation-reduction reactions
- Introduction to electrochemical cells (galvanic and electrolytic cells)
- Cell notation and representation of electrochemical cells
- Standard Electrode Potential and Cell Potential:
- Standard electrode potential and its determination
- Calculation of cell potential using standard electrode potentials
- Relation between cell potential and thermodynamics of the reaction
- Nernst Equation and its Applications:
- Derivation and understanding of the Nernst equation
- Calculation of cell potential under non-standard conditions
- pH dependence and concentration cell applications
- Electrolysis and Electrolytic Cells:
- Faraday’s laws of electrolysis and quantitative aspects of electrolysis
- Electrolytic conductance and its measurement
- Industrial applications of electrolysis (electroplating, electrorefining)
- Conductivity and Ionic Equilibria:
- Introduction to conductivity and conductance measurements
- Kohlrausch’s law and applications
- Ionic equilibria and pH calculations in aqueous solutions
- Corrosion and Corrosion Prevention:
- Understanding the process of corrosion and factors influencing corrosion
- Types of corrosion and their prevention methods
- Cathodic protection and corrosion inhibitors
- Batteries and Fuel Cells:
- Principles of electrochemical energy conversion
- Classification and working principles of batteries
- Introduction to fuel cells and their applications
- Electrochemical Analysis:
- Introduction to voltammetry and potentiometry techniques
- Applications of electrochemical methods in chemical analysis
- Ion-selective electrodes and their applications
The syllabus will often include theoretical concepts, equations, and principles related to electrochemistry. Practical aspects such as laboratory experiments, demonstrations, and data analysis may also be covered to reinforce the understanding of electrochemical principles.
It’s important to note that the specific content and depth of coverage may vary depending on the educational institution, course level, and curriculum guidelines. It is recommended to refer to the official syllabus provided by your educational institution or course instructor for the most accurate and detailed information regarding the Electrochemistry syllabus.
Production of Chemistry syllabus Electrochemistry
The production of a Chemistry syllabus for Electrochemistry typically involves a collaborative effort among educational institutions, curriculum developers, subject experts, and educational boards. Here is a general outline of the process involved in producing a Chemistry syllabus for Electrochemistry:
- Curriculum Development Committee: A committee of subject matter experts, educators, and curriculum developers is formed to design and develop the Chemistry syllabus. The committee may include representatives from educational institutions, professional societies, and examination boards.
- Needs Assessment: The committee identifies the educational goals, learning outcomes, and competencies that students should achieve through the study of Electrochemistry. They consider factors such as the target audience (e.g., high school, undergraduate), educational standards, and the intended application of the syllabus (e.g., entrance examinations, academic programs).
- Content Selection and Organization: The committee determines the key topics and subtopics to be included in the syllabus. They consider the relevance of each topic to Electrochemistry, the level of difficulty, and the sequencing of concepts for effective learning progression. The syllabus should cover fundamental principles, theories, applications, and problem-solving skills related to Electrochemistry.
- Alignment with Educational Standards: The committee ensures that the syllabus aligns with relevant educational standards and guidelines established by educational boards or institutions. This ensures consistency and compatibility with broader educational frameworks.
- Practical Applications and Experiments: The syllabus may include laboratory experiments, practical demonstrations, and hands-on activities to reinforce theoretical concepts and develop practical skills related to Electrochemistry. The committee selects appropriate experiments and provides guidelines for their implementation.
- Assessment and Evaluation: The committee designs assessment methods, including examinations, assignments, and practical assessments, to evaluate students’ understanding of Electrochemistry. The assessment methods should align with the learning outcomes and provide a comprehensive evaluation of students’ knowledge and skills in Electrochemistry.
- Review and Feedback: The draft syllabus is reviewed by subject matter experts, educators, and stakeholders for feedback and suggestions. Revisions are made based on the feedback to ensure accuracy, clarity, and coherence of the syllabus.
- Finalization and Implementation: After incorporating necessary revisions and feedback, the final version of the syllabus is produced. It is then disseminated to educational institutions, teachers, and students for implementation in the classroom.
It’s important to note that the specific process and stakeholders involved may vary depending on the educational system, curriculum development guidelines, and educational policies of each country or educational board. The process described above provides a general overview of the steps involved in producing a Chemistry syllabus for Electrochemistry.
Case Study on Chemistry syllabus Electrochemistry
Case Study: Electrochemical Energy Storage Systems for Renewable Integration
Introduction: Renewable energy sources such as solar and wind power are crucial in reducing greenhouse gas emissions and addressing climate change. However, their intermittent and unpredictable nature poses challenges for their integration into the electrical grid. Electrochemical energy storage systems, based on principles of electrochemistry, offer a viable solution to mitigate the variability of renewable energy sources and provide a stable and reliable electricity supply. This case study examines the application of electrochemistry in energy storage systems for renewable integration.
Background: A utility company, PowerTech Inc., operates a power grid that incorporates a significant amount of renewable energy generation. They face challenges in matching electricity supply with demand due to the intermittent nature of solar and wind power. To address this issue, they decide to implement an electrochemical energy storage system.
Solution: PowerTech Inc. invests in a large-scale electrochemical energy storage system based on lithium-ion battery technology. The system consists of multiple battery banks connected to the grid. During periods of excess renewable energy generation, the excess electricity is used to charge the batteries. Conversely, during periods of high demand or low renewable energy generation, the stored energy in the batteries is discharged to the grid.
Key Steps and Considerations:
- System Sizing and Design:
- PowerTech Inc. assesses the power and energy requirements of the grid to determine the appropriate capacity of the energy storage system.
- They consider factors such as the maximum power output, duration of discharge, and overall energy storage capacity needed.
- Battery Selection:
- PowerTech Inc. evaluates different battery technologies and selects lithium-ion batteries for their high energy density, efficiency, and proven reliability.
- They consider factors such as cycle life, voltage characteristics, safety features, and cost.
- Charging and Discharging Strategy:
- A sophisticated control system is implemented to manage the charging and discharging of the batteries.
- The control system monitors real-time renewable energy generation, grid demand, and battery state of charge to optimize the operation of the energy storage system.
- Integration with the Grid:
- PowerTech Inc. ensures seamless integration of the energy storage system with the existing power grid infrastructure.
- They establish protocols and communication interfaces to enable effective coordination between the energy storage system and the grid operator.
- Monitoring and Maintenance:
- Regular monitoring and maintenance of the energy storage system are performed to ensure optimal performance and longevity.
- PowerTech Inc. employs advanced monitoring systems to track battery health, performance, and any potential issues that may require maintenance or replacement.
Results and Benefits:
- Grid Stability and Reliability:
- The energy storage system helps to balance the supply and demand of electricity, thereby improving grid stability and reliability.
- It smooths out fluctuations in renewable energy generation and provides a stable source of power during periods of low generation or high demand.
- Renewable Energy Integration:
- The energy storage system enables increased penetration of renewable energy sources by storing excess energy and dispatching it when needed.
- This reduces curtailment of renewable energy and enhances grid flexibility and resilience.
- Ancillary Services and Revenue Generation:
- PowerTech Inc. leverages the capabilities of the energy storage system to provide ancillary services to the grid, such as frequency regulation and voltage support.
- By participating in grid ancillary markets, they generate additional revenue streams and improve the overall economics of the energy storage system.
Conclusion: Electrochemical energy storage systems play a vital role in enabling the integration of renewable energy sources into the electrical grid. This case study demonstrates how PowerTech Inc. successfully implemented an electrochemical energy storage system based on lithium-ion batteries to address the challenges of renewable energy integration. The system improves grid stability, facilitates higher penetration of renewable energy, and provides a reliable and sustainable electricity supply. By storing excess renewable energy during periods of high generation and discharging it when needed, the energy storage system helps to balance the supply and demand, reducing the reliance on conventional power sources and minimizing greenhouse gas emissions.
The integration of electrochemical energy storage systems also enhances the flexibility and resilience of the grid. It mitigates the variability of renewable energy sources, smoothing out fluctuations in power output and ensuring a stable and continuous electricity supply. This stability is essential for meeting the growing energy demands of modern society and supporting the transition to a clean energy future.
Furthermore, the energy storage system provides ancillary services to the grid, such as frequency regulation and voltage support, contributing to grid stability and reliability. PowerTech Inc. leverages these capabilities to participate in ancillary markets, generating additional revenue streams and improving the overall economics of the energy storage system.
As the renewable energy sector continues to expand, the role of electrochemical energy storage becomes increasingly crucial. Ongoing research and development efforts are focused on improving the performance, efficiency, and cost-effectiveness of energy storage technologies. Advancements in battery chemistries, materials, and manufacturing processes are driving the evolution of electrochemical energy storage systems, making them more accessible and widely applicable.
To fully realize the potential of electrochemistry in energy storage, collaboration between industry, academia, and policymakers is essential. Continued investment in research and development, supportive policies and regulations, and incentives for the deployment of energy storage systems will accelerate the adoption of electrochemical technologies and pave the way for a sustainable and resilient energy future.
By embracing the potential of electrochemistry in energy storage, PowerTech Inc. and other stakeholders in the energy sector are contributing to a cleaner, more sustainable world. The successful implementation of electrochemical energy storage systems demonstrates their commitment to reducing carbon emissions, fostering renewable integration, and building a resilient and efficient electrical grid. With continued innovation and collaboration, electrochemistry will continue to drive the transformation of the energy sector, powering a sustainable future for generations to come.
White paper on Chemistry syllabus Electrochemistry
Title: Advancing Energy Storage: The Role of Electrochemistry in a Sustainable Future
Abstract: This white paper explores the pivotal role of electrochemistry in advancing energy storage technologies for a sustainable future. With the increasing demand for clean energy sources and the need to address global climate change, electrochemical energy storage systems offer promising solutions for efficient energy management, renewable integration, and decarbonization. This paper provides an overview of key electrochemical concepts, discusses the current state of energy storage technologies, highlights recent advancements, and outlines future research directions to accelerate the adoption of electrochemistry in achieving a sustainable energy landscape.
- Introduction
- The global energy landscape and the need for sustainable solutions
- The significance of electrochemistry in energy storage
- Fundamentals of Electrochemistry
- Redox reactions and electrochemical cells
- Thermodynamics and kinetics of electrochemical processes
- Electrode/electrolyte interfaces and double-layer capacitance
- Energy Storage Technologies
- Overview of different energy storage systems (batteries, supercapacitors, fuel cells)
- Comparative analysis of electrochemical energy storage technologies
- Advantages and limitations of various electrochemical systems
- Electrochemical Battery Technologies
- Lithium-ion batteries and their advancements
- Beyond lithium-ion: emerging battery chemistries (solid-state batteries, metal-air batteries, etc.)
- Innovations in battery materials and designs
- Supercapacitors and Capacitive Energy Storage
- Principles of supercapacitors and their unique advantages
- Advances in electrode materials and capacitive storage mechanisms
- Hybrid systems: combining batteries and supercapacitors for improved performance
- Electrocatalysis and Fuel Cells
- Overview of fuel cell technologies and their applications
- Advances in electrocatalysts for efficient energy conversion
- Solid oxide fuel cells and proton exchange membrane fuel cells
- Integration of Renewables and Grid Applications
- Energy storage for renewable energy integration and grid stabilization
- Role of electrochemical systems in microgrids and smart grids
- Ancillary services and grid-scale applications of electrochemical storage
- Sustainable Materials and Manufacturing
- Environmentally friendly materials for energy storage devices
- Recycling and second-life applications of electrochemical systems
- Sustainable manufacturing processes and circular economy principles
- Challenges and Future Perspectives
- Cost reduction and scalability of electrochemical systems
- Safety and reliability considerations in large-scale energy storage
- Research and development priorities for advancing electrochemistry
- Conclusion
- The transformative potential of electrochemical energy storage
- Collaboration and policy measures for accelerated adoption
- The path forward towards a sustainable energy future
This white paper aims to provide a comprehensive understanding of electrochemistry’s significance in the field of energy storage and its crucial role in transitioning towards a sustainable energy landscape. It emphasizes the need for continued research, technological advancements, and policy support to unlock the full potential of electrochemical energy storage systems.