Electrolysis
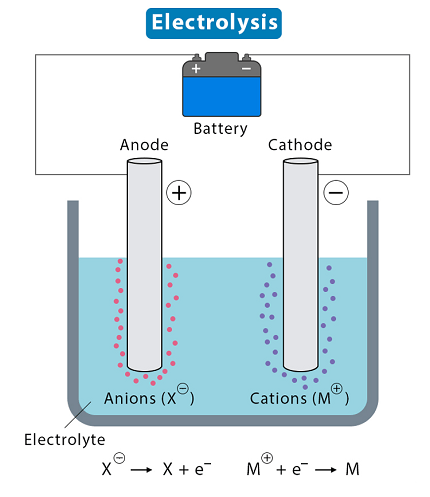
Electrolysis is a chemical process that uses an electric current to bring about a non-spontaneous chemical reaction. It involves the decomposition of an electrolyte, which is a substance that conducts electricity when dissolved or molten, into its constituent ions.
During electrolysis, two electrodes are placed in the electrolyte solution or molten electrolyte. The electrode connected to the positive terminal of the power source is called the anode, while the electrode connected to the negative terminal is called the cathode.
When the electric current passes through the electrolyte, positive ions are attracted to the cathode, where they gain electrons and are reduced. Negative ions, on the other hand, are attracted to the anode, where they lose electrons and are oxidized. This results in the formation of new substances at the electrodes.
Faraday’s laws of electrolysis, formulated by Michael Faraday, describe the relationship between the amount of substance produced or consumed during electrolysis and the amount of electric charge passed through the electrolyte.
Electrolysis has various applications in different industries. For example, it is used in electroplating to deposit a layer of metal onto a surface, in the production of chemicals like chlorine and sodium hydroxide, and in the extraction of metals from their ores.
Overall, electrolysis is an important process in chemistry that enables the manipulation of chemical reactions using electrical energy.
The Chemistry syllabus for the Advanced Course at AIIMS includes the topic of Electrolysis. Electrolysis is the process of using an electric current to drive a non-spontaneous chemical reaction. It involves the decomposition of an electrolyte into its constituent ions when an electric current is passed through it. Important concepts covered in the syllabus include Faraday’s laws of electrolysis, electrolytic cells, electrolysis of molten salts, electrolysis of aqueous solutions, and applications of electrolysis in industries.
What is Required Chemistry syllabus Electrolysis
The required Chemistry syllabus for studying electrolysis typically includes the following key topics:
- Electrolytes: Definition of electrolytes and their classification into strong and weak electrolytes. Understanding the dissociation of electrolytes into ions when dissolved in water or molten state.
- Electrodes: Introduction to electrodes used in electrolysis, such as the anode (positive electrode) and cathode (negative electrode). Understanding their roles in attracting ions and facilitating the redox reactions.
- Electrolytic Cells: Understanding the setup of an electrolytic cell, including the placement of electrodes in the electrolyte and the connection to a power source (battery or power supply).
- Faraday’s Laws of Electrolysis: Understanding and application of Faraday’s laws, which relate the amount of substance produced or consumed during electrolysis to the quantity of electric charge passed through the electrolyte. These laws include Faraday’s First Law (the amount of substance liberated is directly proportional to the quantity of electricity passed) and Faraday’s Second Law (the amounts of different substances liberated by the same quantity of electricity are in the ratio of their respective chemical equivalent weights).
- Electrolysis of Molten Salts: Study of the electrolysis of molten salts, such as sodium chloride (NaCl) and calcium chloride (CaCl2). Understanding the reactions occurring at the anode and cathode, as well as the products formed.
- Electrolysis of Aqueous Solutions: Investigation of the electrolysis of aqueous solutions, including dilute and concentrated solutions of various compounds. Analysis of the different reactions occurring at the anode and cathode, with consideration of the presence of water and the relative reactivity of ions.
- Applications of Electrolysis: Examination of practical applications of electrolysis in various industries, such as electroplating, electrolytic refining of metals, production of chlorine and hydrogen gas, and electrochemical cells.
It is important to note that the specific details of the electrolysis syllabus may vary depending on the educational institution and curriculum. It is advisable to refer to the prescribed textbooks or curriculum guidelines provided by the educational institution for a comprehensive understanding of the required syllabus.
When is Required Chemistry syllabus Electrolysis
The required Chemistry syllabus for electrolysis is typically covered in high school or secondary school level chemistry courses. It is a fundamental topic in the study of electrochemistry and is often included in introductory chemistry curricula.
The exact timing of when electrolysis is taught can vary between educational systems and institutions. However, electrolysis is commonly covered after students have gained a basic understanding of chemical reactions, redox reactions, and ionic compounds.
In most cases, electrolysis is part of a broader unit on electrochemistry, which may also cover concepts such as oxidation-reduction reactions, galvanic cells, and electrochemical cells.
It is best to consult the specific curriculum or syllabus of your educational institution to determine the exact timing and order of topics covered in the Chemistry course, including electrolysis.
Where is Required Chemistry syllabus Electrolysis
The required Chemistry syllabus that includes electrolysis is typically found in the curriculum of high school or secondary school level chemistry courses. The specific location of the syllabus may vary depending on the educational system and institution.
In most cases, electrolysis is a topic covered within a unit or chapter on electrochemistry. This unit may be located within a broader section on chemical reactions or may be specifically designated as a separate section on electrochemistry.
The syllabus for electrolysis will typically outline the key concepts, theories, and practical applications related to electrolysis. It may include topics such as electrolytes, electrodes, electrolytic cells, Faraday’s laws of electrolysis, electrolysis of molten salts, electrolysis of aqueous solutions, and the applications of electrolysis in various industries.
To find the specific location of the electrolysis syllabus within your Chemistry curriculum, you can refer to the course outline, textbook, or curriculum guidelines provided by your educational institution. These resources should provide a detailed breakdown of the topics covered and the order in which they are presented.
How is Required Chemistry syllabus Electrolysis
The required Chemistry syllabus for electrolysis is typically taught through a combination of theoretical concepts, demonstrations, and practical experiments. Here’s a general outline of how the electrolysis syllabus is usually covered:
- Introduction to Electrolysis: The topic begins with an introduction to electrolysis, explaining the basic principles and the purpose of using an electric current to drive chemical reactions.
- Electrolytes and Ionization: Students learn about electrolytes and their ionization behavior in water or when in a molten state. They understand that electrolytes dissociate into positive and negative ions, enabling the conduction of electricity.
- Electrodes and Electrolytic Cells: The role of electrodes, including the anode and cathode, is explained. Students learn about the setup of an electrolytic cell, including the placement of electrodes in the electrolyte solution and the connection to a power source.
- Faraday’s Laws of Electrolysis: Students study Faraday’s laws, which describe the quantitative relationship between the amount of substance produced or consumed during electrolysis and the amount of electric charge passed through the electrolyte. These laws help students understand the stoichiometry of electrolysis reactions.
- Electrolysis of Molten Salts: The electrolysis of molten salts is covered, focusing on specific examples such as the electrolysis of sodium chloride (NaCl) or copper(II) sulfate (CuSO4). Students learn about the reactions occurring at the anode and cathode, as well as the products formed.
- Electrolysis of Aqueous Solutions: Students explore the electrolysis of aqueous solutions, including dilute and concentrated solutions of various compounds. They examine the different reactions occurring at the anode and cathode, considering factors such as the presence of water and the relative reactivity of ions.
- Applications of Electrolysis: The practical applications of electrolysis are discussed, including electroplating, electrolytic refining of metals, production of chlorine and hydrogen gas, and electrochemical cells. Students understand how electrolysis is used in these industrial processes and its significance in various fields.
Throughout the syllabus, students are typically engaged in hands-on activities, demonstrations, and laboratory experiments to reinforce their understanding of electrolysis. These practical sessions allow them to observe the effects of electrolysis and apply theoretical concepts to real-world scenarios.
It’s important to note that the specific teaching methods and order of topics may vary between educational institutions and teachers. Therefore, it’s recommended to refer to the course materials and guidelines provided by your educational institution for a detailed understanding of how the required syllabus on electrolysis is covered in your specific curriculum.
Production of Chemistry syllabus Electrolysis
The production of the Chemistry syllabus for electrolysis involves a careful selection and organization of topics to ensure a comprehensive understanding of the subject. Here’s a general overview of the process involved in developing the syllabus:
- Curriculum Design: Curriculum designers, which may include educators, subject matter experts, and curriculum specialists, review educational standards and guidelines to determine the scope and sequence of the Chemistry syllabus. They consider the level of the course (e.g., high school, secondary school) and the desired learning outcomes.
- Identifying Learning Objectives: The next step involves identifying the specific learning objectives or goals for the electrolysis topic. These objectives outline the knowledge, skills, and understanding that students should acquire through the syllabus. For electrolysis, the objectives may include understanding the principles of electrolysis, applying Faraday’s laws, and recognizing the applications of electrolysis.
- Content Selection: Based on the learning objectives, the curriculum designers select the key topics and concepts that need to be covered in the syllabus. These may include electrolytes, electrodes, electrolytic cells, Faraday’s laws of electrolysis, electrolysis of molten salts, electrolysis of aqueous solutions, and applications of electrolysis.
- Sequence and Organization: The selected topics are then sequenced and organized in a logical manner, ensuring a progressive flow of concepts and building upon prior knowledge. The order of topics may vary, but typically it starts with fundamental concepts and gradually progresses to more complex applications.
- Practical Considerations: Practical considerations are taken into account during the syllabus production. The curriculum designers consider the available resources, time constraints, and the suitability for classroom demonstrations and laboratory experiments. They aim to strike a balance between theoretical understanding and hands-on experiences.
- Assessment Methods: The syllabus may outline the types of assessments that will be used to evaluate students’ understanding of electrolysis. These assessments may include written exams, quizzes, practical experiments, and projects that assess theoretical knowledge, problem-solving skills, and practical application of electrolysis principles.
- Review and Refinement: The syllabus is reviewed and refined through collaboration with other educators, subject experts, and stakeholders. Feedback is incorporated to ensure clarity, accuracy, and alignment with educational standards.
It’s important to note that the production of the Chemistry syllabus for electrolysis can vary between educational systems, institutions, and jurisdictions. The process described here provides a general framework, but specific details may differ based on local curriculum guidelines and requirements.
Case Study on Chemistry syllabus Electrolysis
Sure! Let’s consider a case study on the application of electrolysis in the electroplating industry.
Case Study: Electroplating Process
Electroplating is a widely used industrial process that utilizes electrolysis to deposit a thin layer of metal onto the surface of an object. This case study will focus on the electroplating of a metal object with a layer of silver.
Introduction:
The case study begins with an overview of the electroplating process and its significance in various industries. It explains how electrolysis is employed to achieve the deposition of a metal layer and enhance the properties of the plated object.
Objectives:
The objectives of the case study include understanding the principles of electroplating, analyzing the role of electrolysis in the process, and evaluating the benefits and applications of electroplated objects.
Electrolyte Solution:
The case study explains the composition of the electrolyte solution used in the electroplating process. It typically consists of a silver salt, such as silver nitrate (AgNO3), dissolved in water. The concentration of the electrolyte and the addition of other chemicals for control and improvement are discussed.
Electrodes and Electrolytic Cell:
The setup of the electrolytic cell for electroplating is explained. The metal object to be plated is connected to the cathode, while a silver electrode (usually a silver bar or plate) is connected to the anode. The case study highlights the role of each electrode in attracting ions and facilitating the redox reactions.
Electrolysis Process:
The electrolysis process for electroplating is detailed step-by-step. It includes the application of a direct current (DC) power source, which causes the silver ions (Ag+) in the electrolyte to be attracted to the metal object (cathode). At the cathode, the silver ions gain electrons and are reduced to form a layer of metallic silver on the surface of the object.
Factors Affecting Electroplating:
The case study examines the factors that influence the electroplating process. This includes the voltage and current used, the duration of electrolysis, the concentration of the electrolyte solution, and the cleanliness and preparation of the object’s surface. The importance of these factors in achieving a uniform and adherent metal layer is discussed.
Benefits and Applications:
The case study highlights the benefits and applications of electroplating. It emphasizes how electroplating can improve the appearance, corrosion resistance, and wear resistance of objects. Examples of industries and products that extensively use electroplated objects, such as jewelry, automotive parts, and electronic components, are provided.
Safety and Environmental Considerations:
The case study addresses the safety measures and environmental considerations associated with electroplating. This includes the use of protective equipment, proper handling and disposal of chemicals, and the implementation of waste treatment methods to minimize environmental impact.
Conclusion:
The case study concludes by summarizing the key points discussed and emphasizing the importance of electrolysis in the electroplating process. It highlights the significance of electroplating in various industries and encourages further exploration and research in the field.
Note: This case study provides a brief overview of the electroplating process and can be expanded upon with more detailed information and real-life examples from the electroplating industry. The specific content and structure of the case study can be adapted to meet the educational goals and requirements of the target audience.
White paper on Chemistry syllabus Electrolysis
Title: Advancements and Applications of Electrolysis: A White Paper
Abstract:
This white paper aims to explore the advancements, applications, and future prospects of electrolysis as a versatile chemical process. Electrolysis plays a crucial role in various industries, ranging from metal extraction and electroplating to energy storage and water treatment. This paper provides an overview of the principles, methods, and emerging technologies related to electrolysis. It also discusses the environmental and economic implications of electrolysis and highlights its potential for sustainable and clean energy solutions.
Introduction
1.1 Definition and Principles of Electrolysis
1.2 Historical Background
1.3 Importance of Electrolysis in Modern Industries
Electrolysis Techniques and Methods
2.1 Aqueous Electrolysis
2.2 Molten Salt Electrolysis
2.3 Solid Oxide Electrolysis
2.4 Polymer Electrolyte Membrane Electrolysis
2.5 High-Temperature Electrolysis
2.6 Photoelectrochemical Cells
Faraday’s Laws and Electrochemical Equivalents
3.1 Faraday’s First Law of Electrolysis
3.2 Faraday’s Second Law of Electrolysis
3.3 Calculation of Electrochemical Equivalents
Industrial Applications of Electrolysis
4.1 Metal Extraction and Refining
4.2 Electroplating and Surface Coatings
4.3 Water Electrolysis for Hydrogen Production
4.4 Electrosynthesis of Chemicals
4.5 Electrolytic Cells for Energy Storage
4.6 Water Treatment and Desalination
Advancements in Electrolysis Technologies
5.1 Catalysts and Electrode Materials
5.2 Electrocatalysis and Efficiency Enhancement
5.3 Electrowinning and Electrorefining Processes
5.4 Electrosynthesis of High-Value Chemicals
5.5 Electrosynthesis in Flow Reactors
5.6 Electrification of Industrial Processes
Environmental Implications and Sustainability
6.1 Electrolysis as a Green Process
6.2 Energy Efficiency and Carbon Footprint
6.3 Water Management and Waste Treatment
6.4 Electrolysis for Renewable Energy Integration
Economic Considerations and Market Trends
7.1 Cost Analysis of Electrolysis Systems
7.2 Government Policies and Incentives
7.3 Market Growth and Commercialization
7.4 Electrolysis in the Context of the Energy Transition
Future Outlook and Challenges
8.1 Electrification of Transportation and Power Sectors
8.2 Electrocatalysis and Materials Innovation
8.3 Integration with Renewable Energy Sources
8.4 Scalability and Cost Reduction
8.5 Technological and Regulatory Roadblocks
Conclusion
This white paper concludes by summarizing the advancements and applications of electrolysis and highlighting its potential for sustainable and clean technologies. The continued research, development, and collaboration across academia, industry, and governments are crucial to unlocking the full potential of electrolysis in driving the transition towards a low-carbon and resource-efficient future.
Note: This white paper provides an overview of the topic and can be expanded upon with more in-depth analysis, case studies, and references based on specific areas of interest and target audience.