Enzyme Catalysis
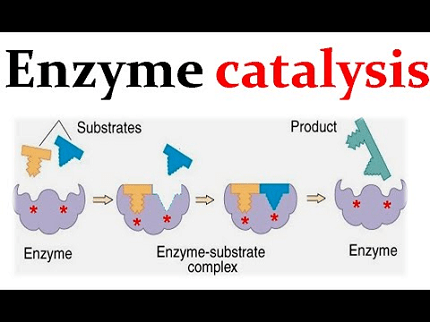
Enzyme catalysis refers to the process by which enzymes accelerate and facilitate chemical reactions in living organisms. Enzymes are specialized proteins that act as biological catalysts, increasing the rate of specific biochemical reactions without being consumed in the process.
Key concepts related to enzyme catalysis include:
- Enzyme structure and function: Enzymes possess a specific three-dimensional structure that allows them to bind to substrates (reactant molecules) and facilitate their conversion into products. The active site of the enzyme is the region where substrate binding and catalysis occur.
- Substrate specificity: Enzymes exhibit high specificity for particular substrates. The shape and chemical properties of the active site enable enzymes to selectively bind and react with specific substrates, ensuring the proper catalytic function.
- Enzyme-substrate complex formation: Enzymes bind to substrates to form an enzyme-substrate complex. This complex undergoes structural changes, facilitating the conversion of substrates into products.
- Catalytic mechanisms: Enzymes utilize various catalytic mechanisms to accelerate reactions. These mechanisms include proximity and orientation effects, acid-base catalysis, covalent catalysis, and metal ion catalysis.
- Enzyme kinetics: The study of enzyme kinetics involves determining the rates of enzyme-catalyzed reactions. Parameters such as the reaction rate, substrate concentration, and enzyme concentration are evaluated to understand the enzymatic reaction mechanisms.
- Factors influencing enzyme activity: Enzyme activity can be influenced by factors such as temperature, pH, substrate concentration, enzyme concentration, and the presence of inhibitors or activators. Optimal conditions are required for enzymes to function efficiently.
- Enzyme regulation: Enzyme activity can be regulated through various mechanisms. This includes allosteric regulation, where regulatory molecules bind to specific sites on the enzyme, influencing its activity. Other modes of regulation include reversible covalent modification and feedback inhibition.
Understanding enzyme catalysis is crucial in many biological processes, including metabolism, signal transduction, and DNA replication. It also has applications in various fields, including medicine, biotechnology, and pharmaceuticals.
What is Required Chemistry syllabus Enzyme Catalysis
The required chemistry syllabus for studying enzyme catalysis typically includes the following topics:
- Introduction to enzymes:
- Definition and characteristics of enzymes
- Importance and role of enzymes in biological systems
- Enzyme structure and function:
- Primary, secondary, tertiary, and quaternary structures of enzymes
- Active site and substrate binding
- Induced fit model
- Enzyme kinetics:
- Rate of enzyme-catalyzed reactions
- Michaelis-Menten equation and derivation
- Enzyme-substrate complex formation
- Determination of kinetic parameters (Vmax, Km, kcat)
- Enzyme mechanisms and catalytic strategies:
- Proximity and orientation effects
- Acid-base catalysis
- Covalent catalysis
- Metal ion catalysis
- Factors influencing enzyme activity:
- Temperature and pH dependence
- Effect of substrate concentration
- Enzyme concentration and saturation kinetics
- Effect of inhibitors and activators
- Enzyme regulation and inhibition:
- Allosteric regulation
- Reversible covalent modification
- Feedback inhibition
- Competitive, non-competitive, and uncompetitive inhibition
- Enzyme kinetics data analysis:
- Lineweaver-Burk plot
- Determination of enzyme inhibition constants (Ki)
It is important to note that the specific content and depth of coverage may vary depending on the educational institution or course. It is recommended to refer to the syllabus provided by the institution or instructor for a more detailed and comprehensive understanding of the enzyme catalysis topic.
When is Required Chemistry syllabus Enzyme Catalysis
The topic of enzyme catalysis is typically covered in advanced-level chemistry courses, such as undergraduate or graduate-level biochemistry or enzymology courses. The specific timing may vary depending on the educational institution and the structure of the curriculum.
In many cases, enzyme catalysis is introduced after covering foundational topics in general chemistry, organic chemistry, and biochemistry. It is common for enzyme catalysis to be taught in the latter part of a biochemistry course, where students have already gained a basic understanding of proteins, enzymes, and enzymatic reactions.
It is advisable to consult the course syllabus or curriculum provided by the educational institution to determine the exact timing and sequencing of enzyme catalysis within the chemistry syllabus.
Where is Required Chemistry syllabus Enzyme Catalysis
The required chemistry syllabus covering enzyme catalysis can typically be found in courses related to biochemistry, enzymology, or advanced chemistry. The specific location within the syllabus may vary depending on the educational institution and the structure of the course.
Enzyme catalysis is often included as a dedicated topic within a larger course on biochemistry. It may be listed as a separate unit or module within the syllabus, along with other related topics such as enzyme kinetics, enzyme regulation, and enzyme mechanisms.
Alternatively, if the course is specifically focused on enzymology, enzyme catalysis may be one of the core concepts covered throughout the duration of the course, with various aspects and subtopics discussed in different sections.
To determine the exact placement of enzyme catalysis within the syllabus, it is recommended to refer to the course syllabus or curriculum provided by the educational institution. This document typically outlines the specific topics, order of presentation, and timeline for the course material.
How is Required Chemistry syllabus Enzyme Catalysis
The required chemistry syllabus for enzyme catalysis is typically taught through a combination of lectures, laboratory experiments, and problem-solving sessions. The specific teaching methods may vary depending on the educational institution and the course structure. Here are some common approaches used to cover enzyme catalysis:
- Lectures: The fundamental concepts of enzyme catalysis, including enzyme structure, kinetics, mechanisms, and regulation, are introduced through lectures. The instructor may use visual aids, models, and case studies to enhance understanding. Lectures provide an overview of the topic and explain the theoretical aspects of enzyme catalysis.
- Laboratory experiments: In some courses, students have the opportunity to perform laboratory experiments related to enzyme catalysis. These experiments allow students to observe enzyme-substrate interactions, measure reaction rates, and study factors affecting enzyme activity. Practical sessions help reinforce theoretical concepts and develop practical skills in experimental techniques.
- Problem-solving sessions: Enzyme catalysis often involves complex mathematical equations and data analysis. Problem-solving sessions are conducted to help students apply concepts learned in lectures and laboratories to solve numerical problems related to enzyme kinetics, enzyme inhibition, and enzyme regulation. These sessions promote critical thinking and reinforce understanding through practice.
- Reading materials and textbooks: Students are typically provided with recommended textbooks and reading materials that cover enzyme catalysis in detail. These resources supplement lectures and provide additional explanations, examples, and case studies for self-study and review.
- Assessments: Assessments, such as quizzes, exams, and assignments, are used to evaluate students’ understanding of enzyme catalysis. These assessments may include theoretical questions, problem-solving exercises, and analysis of experimental data related to enzyme kinetics or enzyme regulation.
It is important to note that the specific approach and emphasis on enzyme catalysis may vary depending on the course level, duration, and the educational institution’s curriculum. The instructor or course syllabus will provide more detailed information on how enzyme catalysis is taught in a particular course.
Case Study on Chemistry syllabus Enzyme Catalysis
Case Study: Enzyme Catalysis in Biotechnology
Introduction: Enzyme catalysis plays a crucial role in various fields, including biotechnology. This case study focuses on the application of enzyme catalysis in the production of a biofuel called biodiesel. Biodiesel is an alternative fuel derived from renewable sources, such as vegetable oils or animal fats. Enzymes, specifically lipases, are used to catalyze the conversion of triglycerides present in these feedstocks into biodiesel.
Case Study Scenario: A biotechnology company has embarked on a project to develop a more efficient and sustainable method for biodiesel production. They aim to utilize enzymes to catalyze the transesterification reaction, which converts triglycerides into biodiesel. Your task is to analyze and optimize the enzyme catalysis process for biodiesel production.
Key Steps and Considerations:
- Enzyme selection: Identify suitable lipase enzymes for the transesterification reaction. Consider factors such as enzyme specificity, stability, and compatibility with the reaction conditions.
- Substrate preparation: Obtain a suitable feedstock, such as vegetable oil or animal fat, rich in triglycerides. Optimize the pretreatment and purification processes to remove impurities and ensure high-quality substrates for the reaction.
- Reaction optimization: a. Reaction conditions: Determine the optimal temperature, pH, and other reaction parameters that maximize the activity and stability of the selected lipase enzyme. b. Co-factors and additives: Investigate the need for any co-factors or additives that enhance enzyme activity and reaction efficiency. c. Enzyme loading and substrate concentration: Optimize the enzyme loading and substrate concentration to achieve the desired conversion rate and maximize the yield of biodiesel.
- Kinetic analysis: Conduct kinetic studies to determine the reaction rate, apparent kinetic constants (e.g., Km and Vmax), and inhibition kinetics if applicable. This information helps understand the enzyme-substrate interactions and assists in optimizing the reaction conditions.
- Enzyme immobilization: Explore the use of immobilized enzymes to improve enzyme stability and facilitate enzyme recovery for reuse. Evaluate different immobilization techniques and matrices for their impact on enzyme activity and stability.
- Process scale-up: Consider the scalability of the enzymatic transesterification process. Evaluate the feasibility of conducting the process on a larger scale while maintaining high conversion rates and minimizing costs.
- Process monitoring and control: Develop methods to monitor the progress of the transesterification reaction, such as measuring the conversion of triglycerides or monitoring the changes in reactant/product concentrations. Implement appropriate process control strategies to optimize reaction parameters in real-time.
Conclusion: Enzyme catalysis plays a vital role in the production of biodiesel, offering advantages such as milder reaction conditions, higher selectivity, and reduced environmental impact compared to traditional chemical catalysts. Through careful enzyme selection, optimization of reaction conditions, and process scale-up considerations, the company can develop an efficient and sustainable enzymatic biodiesel production process. This case study highlights the significance of enzyme catalysis in biotechnology applications and the potential for using enzymes to transform industries toward more environmentally friendly and sustainable practices.
White paper on Chemistry syllabus Enzyme Catalysis
Title: Enzyme Catalysis: Unlocking the Power of Nature’s Catalysts
Abstract: Enzyme catalysis is a remarkable phenomenon that enables living organisms to carry out complex biochemical reactions with exceptional precision and efficiency. This white paper provides an in-depth exploration of enzyme catalysis, including its fundamental principles, mechanisms, and applications across various industries. We delve into the remarkable characteristics of enzymes, their structural and functional aspects, and the intricate interplay between enzymes and substrates. Moreover, we highlight the significance of enzyme catalysis in biotechnology, pharmaceuticals, and environmental sustainability. By harnessing the power of nature’s catalysts, we unlock a world of possibilities for innovation and advancement in diverse scientific domains.
- Introduction
- Definition and significance of enzyme catalysis
- Historical milestones and key discoveries in enzyme research
- Enzyme Structure and Function
- Protein nature of enzymes
- Primary, secondary, tertiary, and quaternary structures
- Active sites and substrate binding
- Induced fit model and specificity
- Enzyme Kinetics and Mechanisms
- Enzyme-substrate complex formation
- Rate equations and Michaelis-Menten kinetics
- Catalytic mechanisms: proximity and orientation effects, acid-base catalysis, covalent catalysis, and metal ion catalysis
- Inhibition and regulation of enzyme activity
- Applications of Enzyme Catalysis
- Biotechnology: Enzymes in industrial processes (e.g., food production, biofuels, detergents)
- Pharmaceuticals: Enzyme inhibition as a therapeutic approach
- Environmental sustainability: Enzymes in waste management and bioremediation
- Engineering Enzymes for Enhanced Catalysis
- Protein engineering approaches (e.g., directed evolution, rational design)
- Tailoring enzymes for specific reactions and improved properties
- Enzyme immobilization and stability enhancement
- Cutting-edge Research and Future Perspectives
- Advances in computational modeling and simulation of enzyme catalysis
- Enzymes in synthetic biology and metabolic engineering
- Emerging trends and challenges in enzyme design and application
- Case Studies
- Case study 1: Enzyme-based biosensors for medical diagnostics
- Case study 2: Enzymatic synthesis of pharmaceutical intermediates
- Case study 3: Enzyme-assisted bioremediation of environmental contaminants
- Conclusion
- Recap of the importance and versatility of enzyme catalysis
- Promising avenues for future research and innovation
- Enzymes as key drivers of sustainable development and technological advancement
In summary, enzyme catalysis represents a remarkable feat of nature that continues to inspire scientists and engineers alike. By understanding the intricate mechanisms and leveraging the catalytic power of enzymes, we open up avenues for groundbreaking discoveries, environmentally friendly processes, and novel solutions to global challenges. Embracing enzyme catalysis as a cornerstone of scientific and technological progress holds immense potential for a sustainable and prosperous future.
