Kohlrausch’s Law
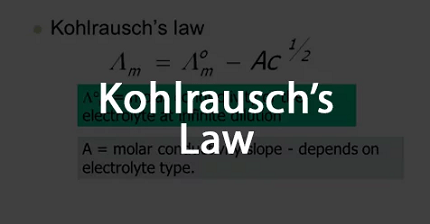
Kohlrausch’s Law is a principle in electrochemistry that describes the relationship between the molar conductivity of an electrolyte and its concentration. The law is named after the German physicist Friedrich Kohlrausch, who formulated it in the late 19th century.
According to Kohlrausch’s Law, the molar conductivity (Λ) of an electrolyte at a given concentration is equal to the sum of the molar conductivities of its constituent ions multiplied by their respective concentration coefficients. Mathematically, it can be represented as:
Λ = Λ₀ + ∑(λᵢ * nᵢ)
Where:
- Λ is the molar conductivity of the electrolyte.
- Λ₀ is the molar conductivity of the solvent (usually water).
- λᵢ is the molar conductivity of the individual ion i.
- nᵢ is the concentration coefficient or number of ions produced by one formula unit of ion i upon dissociation.
Kohlrausch’s Law allows for the determination of molar conductivity values for both strong and weak electrolytes, as it considers the contributions of individual ions in the solution. By measuring the conductivity of different electrolyte concentrations, it is possible to calculate the limiting molar conductivity at infinite dilution (Λ₀) and subsequently obtain other important parameters, such as the degree of dissociation or ionization.
This law has practical applications in various areas of electrochemistry, including the determination of equivalent and molar conductivities, conductometric titrations, and the study of ionic equilibria. It provides insights into the behavior of electrolyte solutions and helps in understanding their electrical conductivity properties.
Kohlrausch’s Law is a concept in electrochemistry that relates to the conductivity of electrolyte solutions. It states that the limiting molar conductivity of an electrolyte can be calculated by taking the sum of the contributions from its individual ions.
In terms of the syllabus for an advanced course in AIIMS (All India Institute of Medical Sciences), the Chemistry syllabus may include the following topics related to Kohlrausch’s Law:
- Electrolytes and Conductivity: Introduction to electrolytes, strong and weak electrolytes, and their behavior in solution. Conductivity and its measurement.
- Ionic Equilibria: The concept of ionic equilibria, including ionization and dissociation of electrolytes. Acid-base equilibria and equilibrium constants.
- Kohlrausch’s Law: Definition and explanation of Kohlrausch’s Law. Understanding the relationship between molar conductivity and concentration of electrolytes.
- Applications of Kohlrausch’s Law: Practical applications of Kohlrausch’s Law, such as the determination of the degree of dissociation of weak electrolytes and the calculation of transport numbers.
- Conductometric Titrations: Introduction to conductometric titrations, including acid-base, redox, and precipitation titrations. Calculation of equivalent and molar conductivities.
- Limitations of Kohlrausch’s Law: Discussing the limitations and assumptions associated with Kohlrausch’s Law, including temperature and concentration effects.
It is important to note that the specific syllabus for AIIMS or any other institution may vary, so it is advisable to consult the official course material or syllabus provided by the institution for accurate and detailed information.
What is Required Chemistry syllabus Kohlrausch’s Law
The required Chemistry syllabus for studying Kohlrausch’s Law typically covers the following topics:
- Electrolytes and Conductivity:
- Introduction to electrolytes and their classification as strong and weak electrolytes.
- Conductivity and its measurement using conductance cells and conductivity meters.
- Relationship between conductivity, resistance, and conductance.
- Ionic Equilibria:
- Ionization and dissociation of electrolytes.
- Acid-base equilibria and equilibrium constants.
- Calculation of pH for strong and weak acids and bases.
- Buffer solutions and their properties.
- Kohlrausch’s Law:
- Definition and explanation of Kohlrausch’s Law.
- Relationship between molar conductivity and concentration of electrolytes.
- Calculation of limiting molar conductivity at infinite dilution (Λ₀).
- Applications of Kohlrausch’s Law:
- Determination of the degree of dissociation of weak electrolytes using conductivity measurements.
- Calculation of transport numbers for individual ions.
- Use of Kohlrausch’s Law in the study of ionic equilibria and conductometric titrations.
- Limitations of Kohlrausch’s Law:
- Temperature and concentration effects on conductivity and molar conductivity.
- Deviations from ideal behavior and associated factors.
It is important to note that the specific syllabus and depth of coverage may vary depending on the educational institution and the level of the course. It is advisable to refer to the official syllabus or course material provided by the institution to get detailed and accurate information about the Chemistry syllabus for studying Kohlrausch’s Law.
When is Required Chemistry syllabus Kohlrausch’s Law
The Required Chemistry syllabus that includes Kohlrausch’s Law is typically covered in higher-level courses in physical or analytical chemistry. It is commonly included in the syllabus of undergraduate or postgraduate programs in chemistry or related fields. The exact timing of when Kohlrausch’s Law is taught may vary depending on the curriculum structure of the educational institution.
In many cases, Kohlrausch’s Law is introduced after covering foundational topics in general chemistry, such as stoichiometry, chemical equilibrium, and acid-base concepts. It is often covered alongside other topics related to electrolytes, conductivity, and ionic equilibria. This allows students to have a solid understanding of these fundamental concepts before delving into the applications and principles of Kohlrausch’s Law.
It is important to consult the specific syllabus or course outline provided by your educational institution to determine the exact timing of when Kohlrausch’s Law is included in the chemistry curriculum. This will ensure that you have accurate information regarding the scheduling and sequencing of topics covered in your particular academic program.
Where is Required Chemistry syllabus Kohlrausch’s Law
The Required Chemistry syllabus that includes Kohlrausch’s Law can be found in various educational settings, including universities, colleges, and institutions offering chemistry programs. The syllabus is typically part of advanced courses in physical chemistry, analytical chemistry, or electrochemistry.
The syllabus is usually provided by the respective educational institution and can be accessed through the chemistry department or faculty. It may be available in the form of a course outline, curriculum document, or a detailed syllabus provided by the instructor or department.
To locate the specific Chemistry syllabus that includes Kohlrausch’s Law, you can follow these steps:
- Check the official website of your educational institution: Visit the website of the university, college, or institution where you are studying or planning to study chemistry. Look for the department or faculty of chemistry, and navigate to their webpage.
- Explore the Chemistry department’s webpage: On the department’s webpage, you may find information about the curriculum, courses offered, and course descriptions. Look for information related to physical chemistry, analytical chemistry, or electrochemistry courses.
- Contact the Chemistry department: If you are unable to find the syllabus online, consider contacting the Chemistry department directly. They can provide you with the required information, including the syllabus or course outline that covers Kohlrausch’s Law.
It is important to note that the availability and accessibility of the syllabus may vary depending on the educational institution and the specific course you are enrolled in or planning to take.
How is Required Chemistry syllabus Kohlrausch’s Law
The Required Chemistry syllabus that includes Kohlrausch’s Law is typically structured to provide students with a comprehensive understanding of the topic. The syllabus is designed to cover the theoretical concepts, practical applications, and related topics associated with Kohlrausch’s Law. While the specific structure may vary depending on the educational institution and course level, the syllabus generally follows a logical progression. Here is a general outline of how the syllabus on Kohlrausch’s Law may be organized:
- Introduction to Electrolytes and Conductivity:
- Definition and classification of electrolytes as strong and weak.
- Conductivity and its measurement using conductance cells or conductivity meters.
- Factors influencing the conductivity of electrolyte solutions.
- Ionic Equilibria:
- Dissociation and ionization of electrolytes in solution.
- Acid-base equilibria, including the concept of pH and equilibrium constants.
- Buffer solutions and their properties.
- Kohlrausch’s Law:
- Definition and formulation of Kohlrausch’s Law.
- Understanding the relationship between molar conductivity and concentration of electrolytes.
- Calculation of limiting molar conductivity at infinite dilution (Λ₀).
- Applications of Kohlrausch’s Law:
- Determination of the degree of dissociation of weak electrolytes using conductivity measurements.
- Calculation of transport numbers for individual ions.
- Use of Kohlrausch’s Law in the study of ionic equilibria and conductometric titrations.
- Limitations and Deviations:
- Discussion on the limitations of Kohlrausch’s Law, such as temperature and concentration effects.
- Explanation of deviations from ideal behavior in certain electrolyte systems.
- Experimental Techniques:
- Conductivity measurement techniques and instrumentation.
- Laboratory experiments related to Kohlrausch’s Law and conductivity measurements.
It’s important to note that the actual syllabus content and organization may vary depending on the institution and specific course. The above outline provides a general overview of the topics that are typically covered when studying Kohlrausch’s Law in a required Chemistry syllabus.
Case Study on Chemistry syllabus Kohlrausch’s Law
Title: Application of Kohlrausch’s Law in Determining the Degree of Dissociation of Weak Electrolytes
Introduction: Kohlrausch’s Law is a fundamental concept in electrochemistry that relates to the conductivity of electrolyte solutions. It states that the molar conductivity of an electrolyte can be calculated by summing the contributions from its individual ions. This case study focuses on the application of Kohlrausch’s Law in determining the degree of dissociation of a weak electrolyte through experimental measurements of conductivity.
Objective: The objective of this case study is to utilize Kohlrausch’s Law to determine the degree of dissociation (α) of a weak electrolyte and understand the behavior of weak electrolytes in solution.
Experimental Procedure:
- Preparation of solutions: Prepare solutions of a known concentration of a weak electrolyte (e.g., acetic acid, ethanoic acid) in varying concentrations.
- Measurement of conductivity: Measure the conductivity of each solution using a conductivity meter or conductance cell.
- Determination of molar conductivity: Calculate the molar conductivity (Λ) of each solution by dividing its conductivity by the concentration.
- Determination of limiting molar conductivity: Extrapolate the molar conductivity values to infinite dilution by plotting a graph of Λ vs. √c (square root of concentration) and extrapolating to zero concentration (√c → 0).
- Calculation of degree of dissociation: Use Kohlrausch’s Law to calculate the degree of dissociation (α) using the relationship α = Λ/Λ₀, where Λ₀ is the limiting molar conductivity at infinite dilution.
- Analysis and interpretation: Analyze the data obtained and interpret the degree of dissociation in terms of the weak electrolyte’s behavior in solution.
Results and Discussion:
- Plotting the graph of molar conductivity (Λ) vs. √c should yield a straight line that passes through the origin. The slope of this line is related to the equivalent conductance at infinite dilution (Λ₀).
- The limiting molar conductivity (Λ₀) can be determined by extrapolating the line to √c = 0. This value represents the conductivity contributed solely by the fully dissociated ions in the solution.
- Using Kohlrausch’s Law, the degree of dissociation (α) can be calculated by dividing the molar conductivity (Λ) of the weak electrolyte solution by Λ₀.
- The obtained degree of dissociation provides insights into the extent of ionization of the weak electrolyte and its behavior in solution.
Conclusion: This case study highlights the application of Kohlrausch’s Law in determining the degree of dissociation of a weak electrolyte. By measuring the conductivity of solutions at different concentrations and employing Kohlrausch’s Law, the degree of dissociation can be calculated. This experimental approach allows for a deeper understanding of the behavior of weak electrolytes in solution and their ionization characteristics. The study demonstrates the practical application of Kohlrausch’s Law in the field of electrochemistry and its relevance in determining important parameters of electrolyte solutions.
White paper on Chemistry syllabus Kohlrausch’s Law
Title: Kohlrausch’s Law: Understanding Electrolyte Conductivity and Its Applications
Abstract: Kohlrausch’s Law is a fundamental concept in electrochemistry that provides insights into the conductivity of electrolyte solutions. Developed by Friedrich Kohlrausch in the late 19th century, this law relates the molar conductivity of an electrolyte to the sum of the molar conductivities of its constituent ions. This white paper explores the principles of Kohlrausch’s Law, its underlying theory, experimental applications, and practical significance in various fields of chemistry and beyond.
- Introduction:
- Overview of electrolytes and their behavior in solution.
- Introduction to the concept of electrical conductivity and its measurement.
- Development of Kohlrausch’s Law:
- Historical background and Friedrich Kohlrausch’s contribution.
- Explanation of the law’s formulation and its mathematical representation.
- Principles of Kohlrausch’s Law:
- Detailed explanation of the law’s principles and significance.
- The relationship between molar conductivity and concentration of electrolytes.
- Connection to the concept of limiting molar conductivity at infinite dilution.
- Applications of Kohlrausch’s Law:
- Determination of degree of dissociation of weak electrolytes.
- Conductometric titrations and their practical applications.
- Use in the study of ionic equilibria and pH calculations.
- Industrial applications, such as process control and quality assurance.
- Experimental Techniques and Considerations:
- Conductivity measurement techniques and instruments.
- Factors affecting conductivity measurements, including temperature and concentration.
- Limitations and challenges in applying Kohlrausch’s Law.
- Significance and Impact:
- Importance of Kohlrausch’s Law in understanding electrolyte behavior.
- Contribution to the development of electrochemical theories.
- Influence on various fields, including chemistry, biochemistry, and materials science.
- Future Directions and Advancements:
- Current research and advancements related to Kohlrausch’s Law.
- Integration with modern techniques and technologies.
- Potential for further applications and interdisciplinary collaborations.
- Conclusion:
- Recap of the key concepts and applications of Kohlrausch’s Law.
- Emphasis on its fundamental role in understanding electrolyte conductivity.
- Acknowledgment of its continued relevance in contemporary scientific research.
This white paper aims to provide a comprehensive understanding of Kohlrausch’s Law and its significance in the realm of electrochemistry and related disciplines. By elucidating its principles, applications, and future prospects, this paper seeks to promote further exploration and utilization of this fundamental law in scientific endeavors.
