Laws of Electrolysis
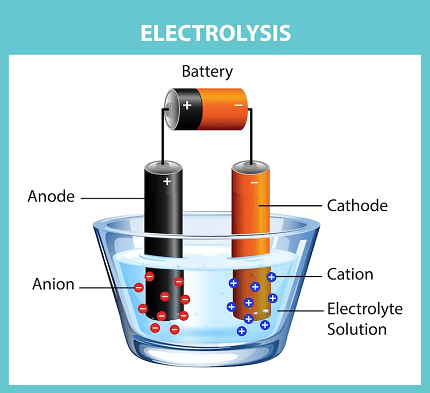
The Laws of Electrolysis are a set of principles formulated by Michael Faraday that describe the quantitative relationships between the amounts of substances involved in an electrolytic reaction. These laws are:
- Faraday’s First Law of Electrolysis: It states that the amount of chemical reaction occurring at an electrode is directly proportional to the quantity of electric charge passed through the electrolyte. Mathematically, it can be expressed as:Amount of substance deposited or liberated = (Current × Time) / Faraday’s constantwhere the Faraday’s constant is the charge carried by one mole of electrons, approximately 96,500 C/mol.
- Faraday’s Second Law of Electrolysis: It states that the amounts of different substances deposited or liberated by the same amount of electric charge passing through the electrolyte are directly proportional to their respective chemical equivalent weights. Mathematically, it can be expressed as:Ratio of the masses of substances deposited or liberated = Ratio of their chemical equivalent weightsThe chemical equivalent weight is the mass of a substance that reacts with or is deposited by one mole of electrons during electrolysis.
These laws provide a basis for understanding and quantifying the electrolytic processes, such as the deposition of metals during electroplating or the decomposition of compounds during electrolysis.
What is Required Chemistry syllabus Laws of Electrolysis
The syllabus for the topic “Laws of Electrolysis” in the chemistry section typically covers the following concepts:
- Electrolytic cells and electrolysis: Understanding the setup and functioning of electrolytic cells, including the electrodes, electrolyte, and external power source.
- Faraday’s laws: A detailed study of Faraday’s First Law, which relates the amount of substance deposited or liberated during electrolysis to the quantity of electric charge passed. Faraday’s Second Law, which relates the amounts of different substances deposited or liberated, should also be covered.
- Electrochemical equivalents: Exploring the concept of electrochemical equivalents, which are the ratios of masses of substances deposited or liberated during electrolysis.
- Calculation problems: Solving numerical problems based on the laws of electrolysis, involving calculations of the amount of substance deposited or liberated, given current, time, and electrode reactions.
It is essential to have a clear understanding of the principles behind the laws of electrolysis, along with the ability to apply them to solve relevant problems.
When is Required Chemistry syllabus Laws of Electrolysis
The Laws of Electrolysis are typically covered in high school or introductory college-level chemistry courses. They are fundamental principles in the field of electrochemistry and are commonly taught as part of the curriculum on redox reactions and electrochemical cells.
In terms of specific educational programs or exams, the timing may vary. In some curricula, the Laws of Electrolysis may be taught as part of a broader unit on electrochemistry, while in others, it may be covered as a separate topic. For entrance exams like AIIMS, which is a medical entrance exam in India, the syllabus typically follows the curriculum of higher secondary education, which would include the Laws of Electrolysis.
To get precise information about when the Laws of Electrolysis are taught in a specific educational program or exam, it is best to consult the official curriculum or syllabus guidelines provided by the respective educational institution or exam conducting authority.
Where is Required Chemistry syllabus Laws of Electrolysis
The Laws of Electrolysis are typically included in the chemistry curriculum for high school or introductory college-level courses. They are a fundamental topic in the field of electrochemistry and are commonly covered as part of the redox reactions and electrochemical cells unit.
In terms of specific educational programs or exams, the inclusion of Laws of Electrolysis in the chemistry syllabus may vary. However, for exams such as AIIMS (All India Institute of Medical Sciences), which is a medical entrance exam in India, the syllabus usually aligns with the curriculum of higher secondary education.
To find the exact placement of the Laws of Electrolysis within the chemistry syllabus, it is advisable to refer to the official curriculum or syllabus guidelines provided by the educational institution or the exam conducting authority. These documents will provide comprehensive and accurate information about the specific topics covered in the chemistry syllabus, including the Laws of Electrolysis.
How is Required Chemistry syllabus Laws of Electrolysis
The Laws of Electrolysis are typically taught through a combination of theoretical concepts, experimental demonstrations, and problem-solving exercises. Here’s how the required chemistry syllabus on the Laws of Electrolysis may be covered:
- Introduction to Electrolysis: The topic begins with an introduction to electrolysis, explaining the principles of electrochemical cells and the process of electrolysis. Students learn about the components of an electrolytic cell, including the electrodes, electrolyte, and external power source.
- Faraday’s First Law: The first law, which states that the amount of substance deposited or liberated during electrolysis is directly proportional to the quantity of electric charge passed, is introduced. Students understand the relationship between current, time, and the amount of substance involved. This includes the use of Faraday’s constant to convert charge into moles of substance.
- Faraday’s Second Law: The second law, stating that the amounts of different substances deposited or liberated are directly proportional to their respective chemical equivalent weights, is explained. Students learn to calculate the ratio of masses of substances involved in an electrolytic reaction based on their chemical equivalents.
- Electrochemical Equivalents: The concept of electrochemical equivalents is explored further, emphasizing the relationship between the amount of substance involved in an electrolysis reaction and the number of moles of electrons transferred during the process. This includes understanding the relationship between the mass of a substance deposited or liberated and the charge passed.
- Problem-Solving and Applications: Students are presented with problem-solving exercises involving electrolysis reactions. These problems require applying Faraday’s laws to calculate the amounts of substances involved, determining the mass of the substance deposited or liberated, and analyzing various electrolytic processes.
Throughout the syllabus, practical demonstrations and experiments may be conducted to reinforce the theoretical concepts and provide hands-on experience with electrolysis. These experiments may involve the use of simple electrolytic cells and measurements of mass changes or gas volume evolved during electrolysis.
It is important for students to understand the underlying principles of the Laws of Electrolysis and develop proficiency in applying them to solve numerical problems and analyze electrolytic processes.
Case Study on Chemistry syllabus Laws of Electrolysis
Case Study: Electroplating Process and the Laws of Electrolysis
Introduction: Electroplating is a common industrial process that utilizes the principles of electrolysis. Let’s consider a case study on the electroplating of a metal object, such as a silver spoon, with a thin layer of gold. In this process, the Laws of Electrolysis play a crucial role.
Scenario: A jewelry manufacturer wants to enhance the appearance of silver spoons by electroplating them with a layer of gold. They need to ensure a consistent and controlled deposition of gold on the spoons to achieve the desired aesthetic effect.
Application of the Laws of Electrolysis:
- Faraday’s First Law: According to this law, the amount of gold deposited on the spoon is directly proportional to the quantity of electric charge passed through the electrolyte. The manufacturer needs to determine the appropriate current and time for the electroplating process to ensure the desired thickness of the gold layer.
- Faraday’s Second Law: This law states that the amounts of different substances deposited or liberated during electrolysis are directly proportional to their respective chemical equivalent weights. In the case of electroplating, the chemical equivalent weight of gold and silver plays a crucial role in determining the relative amounts of gold and silver ions involved in the process.
Experimental Considerations: To achieve successful electroplating, the following experimental considerations should be taken into account:
- Electrolyte Selection: The manufacturer needs to choose an appropriate electrolyte that contains gold ions (Au+) to facilitate the deposition of gold onto the silver spoons. This electrolyte should also provide a stable and consistent plating solution.
- Electrode Selection: The spoons act as the cathode (negative electrode) during electroplating, while a gold anode (positive electrode) is used. The choice of electrode materials and their surface area can influence the deposition rate and quality of the gold layer.
- Current and Time Control: Based on Faraday’s First Law, the manufacturer must carefully control the current passed through the electrolyte and the duration of the electroplating process. This ensures the desired thickness of the gold layer and avoids over-plating or under-plating.
- Monitoring the Process: The manufacturer should continuously monitor the process parameters, such as current, time, and visual inspection of the plated spoons, to ensure uniformity and quality control.
Conclusion: The case study on electroplating demonstrates the practical application of the Laws of Electrolysis. By understanding and applying Faraday’s laws, the jewelry manufacturer can achieve controlled and consistent electroplating of silver spoons with a layer of gold, enhancing their appearance and value. The Laws of Electrolysis provide the scientific foundation for understanding and optimizing various electrochemical processes in industries ranging from electronics to metallurgy.
White paper on Chemistry syllabus Laws of Electrolysis
Title: Understanding the Laws of Electrolysis: Principles, Applications, and Implications
Abstract: Electrolysis is a fundamental process in electrochemistry that has far-reaching applications across various industries. The Laws of Electrolysis, formulated by Michael Faraday, provide the theoretical framework for understanding and quantifying the relationships between electric charge, chemical reactions, and the deposition or liberation of substances during electrolysis. This white paper explores the principles underlying the Laws of Electrolysis, their applications in practical scenarios, and their implications for industries such as electroplating, metal extraction, energy storage, and more. By delving into the fundamental concepts and real-world examples, this paper aims to provide a comprehensive understanding of the Laws of Electrolysis and their significance in modern science and technology.
- Introduction:
- Overview of electrolysis and its significance in various industries.
- Introducing the Laws of Electrolysis and their historical context.
- Faraday’s First Law of Electrolysis:
- Explanation of the first law, which relates the amount of substance deposited or liberated to the quantity of electric charge passed through the electrolyte.
- Mathematical representation and practical implications of the first law.
- Examples and applications of Faraday’s First Law in industries such as electroplating and metal refining.
- Faraday’s Second Law of Electrolysis:
- Explanation of the second law, which establishes the relationship between the amounts of different substances deposited or liberated during electrolysis.
- Understanding chemical equivalent weights and their role in the second law.
- Illustrating the second law through examples from electrochemical processes.
- Electrochemical Equivalents and Quantitative Analysis:
- Definition and significance of electrochemical equivalents.
- Methods for determining electrochemical equivalents experimentally.
- Quantitative analysis of electrolytic processes using Faraday’s laws.
- Applications of the Laws of Electrolysis:
- Electroplating: Understanding how Faraday’s laws govern the controlled deposition of metals onto surfaces.
- Metal Extraction: Exploring the principles behind electrorefining and electrowinning processes.
- Energy Storage: Discussing the role of electrolysis in electrochemical cells and battery technologies.
- Electrolytic Water Splitting: Analyzing the use of Faraday’s laws in the production of hydrogen and oxygen through water electrolysis.
- Implications and Future Directions:
- Implications of the Laws of Electrolysis in terms of industrial processes, environmental impact, and technological advancements.
- Emerging trends and research directions in the field of electrolysis.
- Potential areas of innovation and optimization in electrolytic processes.
- Conclusion:
- Recapitulation of the key principles and applications of the Laws of Electrolysis.
- Emphasizing the importance of understanding Faraday’s laws for advancements in electrochemical science and technology.
By providing a comprehensive overview of the Laws of Electrolysis, this white paper aims to foster a deeper understanding of this foundational topic and inspire further research and innovation in the field of electrochemistry.
