Rate of a reaction
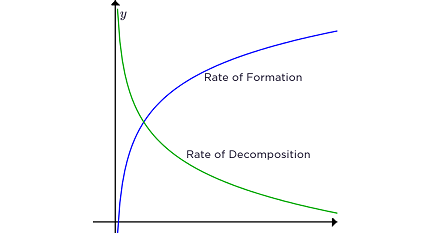
The rate of a reaction refers to how quickly a chemical reaction occurs, specifically the change in concentration of reactants or products over time. It is often expressed as the rate of disappearance of a reactant or the rate of formation of a product.
The rate of a reaction can be determined by measuring the change in concentration of a reactant or product at different time intervals. The rate is typically calculated by dividing the change in concentration by the corresponding change in time.
Factors that influence the rate of a reaction include:
- Concentration: Increasing the concentration of reactants generally increases the reaction rate, as more collisions between particles occur.
- Temperature: Higher temperatures usually lead to faster reaction rates because particles have more energy and move faster, increasing the frequency and effectiveness of collisions.
- Surface area: Breaking a solid reactant into smaller particles increases the surface area available for reactions, leading to a faster rate.
- Catalysts: Catalysts are substances that speed up the rate of a reaction by providing an alternative reaction pathway with lower activation energy. They increase the frequency of effective collisions.
- Pressure (for gases): Increasing the pressure of gaseous reactants can increase the reaction rate by decreasing the volume and increasing the concentration.
The rate of a reaction can be determined experimentally and can be described using a rate law, which relates the rate to the concentrations of reactants. The rate constant (k) is a proportionality constant in the rate law equation and represents the specific reaction rate at a given temperature.
The syllabus for the Advanced Course AIIMS in Chemistry includes the topic of “Rate of a Reaction.” The study of rate of a reaction involves understanding the speed at which a chemical reaction occurs and the factors that influence it. This topic covers concepts such as rate laws, rate constants, reaction order, and the effect of concentration, temperature, and catalysts on reaction rates. Students will also learn about reaction mechanisms and how to determine the rate-determining step in a reaction.
What is Required Chemistry syllabus Rate of a reaction
The required chemistry syllabus for studying the rate of a reaction typically includes the following topics:
- Introduction to rate of reaction: Definition of rate, factors affecting rate, and the importance of studying reaction rates.
- Rate expression and rate law: Understanding the rate law equation that relates the rate of a reaction to the concentrations of reactants. This involves determining the order of the reaction with respect to each reactant and the overall reaction order.
- Rate constant and rate-determining step: Introducing the rate constant (k) as a proportionality constant in the rate law equation and its significance. Exploring the concept of the rate-determining step, which is the slowest step in a reaction mechanism that determines the overall rate.
- Integrated rate laws: Solving differential rate equations and obtaining integrated rate laws for various reaction orders. Understanding the relationship between concentration and time during a reaction.
- Reaction mechanisms: Exploring the steps involved in a reaction mechanism and how they relate to the rate law. Identifying elementary steps, intermediates, and the overall balanced equation.
- Factors affecting reaction rate: Studying the impact of concentration, temperature, surface area, catalysts, and pressure (for gaseous reactions) on reaction rates. Understanding the underlying principles and mechanisms behind these factors.
- Collision theory: Introducing the collision theory, which explains the importance of effective collisions between reacting particles for a reaction to occur. Exploring concepts such as activation energy, transition state, and reaction kinetics.
- Reaction order and rate-determining step determination: Determining the reaction order experimentally by analyzing reaction rate data and plotting concentration-time graphs. Identifying the rate-determining step from experimental observations.
- Reaction kinetics: Understanding the quantitative analysis of reaction rates, including rate constants, reaction orders, and rate laws. Applying mathematical techniques to calculate reaction rates and rate constants.
It’s important to note that the specific topics covered in the syllabus may vary depending on the educational institution and curriculum.
When is Required Chemistry syllabus Rate of a reaction
The topic of rate of a reaction is typically covered in high school or secondary school chemistry courses, as well as introductory college-level chemistry courses. The timing of when it is taught can vary depending on the specific curriculum and educational system. In most cases, the topic of rate of a reaction is introduced after foundational concepts such as chemical equations, stoichiometry, and chemical equilibrium have been covered. It is a fundamental concept in understanding chemical kinetics and is essential for further studies in chemistry, including advanced courses and specialized fields such as chemical engineering and biochemistry.
Where is Required Chemistry syllabus Rate of a reaction
The required chemistry syllabus that includes the topic of rate of a reaction is typically taught in educational institutions such as high schools, secondary schools, and colleges. It is a part of the curriculum in chemistry courses offered at these institutions. The specific placement of the topic within the syllabus may vary depending on the educational system and curriculum structure. In most cases, the topic of rate of a reaction is covered as a part of the broader area of chemical kinetics, which deals with the study of reaction rates, mechanisms, and factors affecting the rates of chemical reactions.
How is Required Chemistry syllabus Rate of a reaction
The required chemistry syllabus for the topic of rate of a reaction is typically taught through a combination of theoretical concepts and practical applications. Here’s an overview of how the syllabus is usually covered:
- Introduction: The syllabus starts with an introduction to the concept of reaction rate, emphasizing its importance and relevance in understanding chemical reactions.
- Rate expression and rate laws: Students learn about rate laws, which mathematically describe the relationship between the rate of a reaction and the concentrations of reactants. They learn how to write and interpret rate expressions and determine the order of a reaction.
- Experimental determination of rate: Students are introduced to experimental techniques used to measure reaction rates. They learn how to collect data and analyze it to determine the rate of a reaction. This involves plotting concentration-time graphs and interpreting the results.
- Rate constant and rate-determining step: The syllabus covers the concept of the rate constant (k) and its significance in the rate law equation. Students learn how to calculate the rate constant and understand its relationship with temperature. The concept of the rate-determining step, which determines the overall rate of a reaction, is also covered.
- Factors affecting reaction rate: Students study the factors that influence the rate of a reaction, including concentration, temperature, surface area, catalysts, and pressure (for gaseous reactions). They learn how to analyze and interpret the impact of these factors on reaction rates.
- Integrated rate laws: Students learn how to solve differential rate equations and obtain integrated rate laws for various reaction orders. They explore the relationship between concentration and time during a reaction.
- Reaction mechanisms: The syllabus covers the concept of reaction mechanisms, which are the step-by-step processes that occur during a chemical reaction. Students learn how to identify elementary steps, intermediates, and the overall balanced equation. They also learn how reaction mechanisms relate to the rate law.
- Collision theory: Students are introduced to the collision theory, which explains how reaction rates depend on the frequency and effectiveness of collisions between reacting particles. They learn about concepts such as activation energy, transition state, and the role of collision geometry.
- Kinetics and equilibrium: The syllabus often covers the relationship between reaction kinetics and chemical equilibrium. Students learn how the rates of forward and reverse reactions influence the overall equilibrium of a reaction.
Throughout the syllabus, students typically engage in problem-solving exercises, numerical calculations, and laboratory experiments to reinforce their understanding of rate of a reaction concepts. Practical applications and real-life examples may also be discussed to highlight the importance of rate of a reaction in various fields, such as industry, medicine, and environmental science.
Production of Chemistry syllabus Rate of a reaction
The production of a chemistry syllabus for the topic of rate of a reaction involves careful consideration of the fundamental concepts and learning objectives related to this subject. The syllabus is typically designed by educational institutions or curriculum development bodies and may vary depending on the specific educational system or level of study. Here are some key aspects that are considered in the production of a chemistry syllabus for the topic of rate of a reaction:
- Learning Outcomes: The syllabus defines the desired learning outcomes for students. These may include understanding the concept of reaction rate, being able to determine rate expressions and rate laws, analyzing experimental data to calculate reaction rates, and explaining the factors that affect the rate of a reaction.
- Conceptual Framework: The syllabus outlines the conceptual framework for the topic. It identifies the key concepts and theories that students should grasp, such as rate laws, rate constants, reaction mechanisms, and factors influencing reaction rates.
- Sequence of Topics: The syllabus establishes the order in which the topics related to rate of a reaction will be presented. This ensures a logical progression of learning, starting from basic concepts and gradually building up to more complex aspects.
- Depth of Coverage: The syllabus specifies the depth of coverage for each topic. It determines the level of detail and understanding required from students, ensuring a comprehensive understanding of the subject matter.
- Practical Applications: The syllabus may include practical applications and real-life examples to illustrate the relevance and importance of rate of a reaction in various fields, such as pharmaceuticals, environmental science, and chemical engineering.
- Teaching Methodology: The syllabus may provide guidelines on teaching methodologies and instructional strategies that can effectively convey the concepts of rate of a reaction. This could include a combination of lectures, demonstrations, problem-solving exercises, and laboratory experiments.
- Assessment Methods: The syllabus may outline assessment methods, such as exams, quizzes, or laboratory reports, that can evaluate students’ understanding and mastery of the topic. It may also specify the weightage or grading criteria for each assessment method.
- Resources and References: The syllabus may suggest textbooks, reference materials, or online resources that can support students’ learning and provide additional information on the topic of rate of a reaction.
The production of a chemistry syllabus for rate of a reaction involves aligning the content with educational standards, considering the needs of the students, and ensuring the syllabus is coherent, structured, and conducive to effective learning.
Case Study on Chemistry syllabus Rate of a reaction
Case Study: Reaction Rate of Hydrogen Peroxide Decomposition
Introduction: The decomposition of hydrogen peroxide (H2O2) is a classic example used to study the rate of a reaction. Hydrogen peroxide decomposes into water (H2O) and oxygen gas (O2), and the reaction rate can be measured by observing the rate of oxygen gas evolution. This case study explores the factors affecting the rate of the hydrogen peroxide decomposition reaction and demonstrates how the rate can be determined experimentally.
Experimental Setup: A common experimental setup for studying the rate of the hydrogen peroxide decomposition reaction involves a reaction vessel connected to a gas collection apparatus. The reaction vessel contains a known volume of hydrogen peroxide solution, and a catalyst, such as manganese dioxide (MnO2), is often used to accelerate the reaction. As the reaction proceeds, oxygen gas is evolved and collected in a graduated cylinder or a gas syringe.
Factors Affecting Reaction Rate:
- Concentration: The rate of the reaction can be affected by the concentration of hydrogen peroxide. By varying the initial concentration of hydrogen peroxide while keeping other factors constant, the effect of concentration on the reaction rate can be investigated.
- Temperature: Temperature has a significant impact on reaction rates. By conducting the reaction at different temperatures, it is possible to observe how the rate changes with temperature. Higher temperatures generally result in faster reaction rates due to increased molecular collisions and greater kinetic energy.
- Catalyst: The presence of a catalyst, such as manganese dioxide, can greatly accelerate the reaction rate. Comparing the reaction rate with and without a catalyst allows for the assessment of its effect on the reaction kinetics.
Experimental Procedure:
- Prepare a series of hydrogen peroxide solutions with different concentrations. This can be achieved by diluting a stock solution with distilled water.
- Set up the reaction vessel and connect it to the gas collection apparatus. Ensure that the gas collection system is airtight.
- Add a small amount of manganese dioxide catalyst to the reaction vessel.
- Pour a specific volume of the hydrogen peroxide solution into the reaction vessel, ensuring all measurements are accurately recorded.
- Start the stopwatch or timer as soon as the hydrogen peroxide solution is added to the reaction vessel.
- Observe the gas evolution over a specific time interval and record the volume of oxygen gas collected at regular time intervals.
- Repeat the experiment with different hydrogen peroxide concentrations and/or at different temperatures to investigate their effects on the reaction rate.
Data Analysis: Using the collected data, a graph of the volume of oxygen gas evolved versus time can be plotted. The slope of the resulting curve represents the reaction rate at different time intervals. By comparing the rates under different conditions, the impact of concentration, temperature, and catalyst can be assessed.
Conclusion: This case study highlights the application of the hydrogen peroxide decomposition reaction to study the rate of a reaction. Through experimental investigation and analysis, it becomes possible to understand how factors like concentration, temperature, and the presence of a catalyst affect the rate of the reaction. Such case studies provide valuable insights into the principles of chemical kinetics and the factors that influence reaction rates.
White paper on Chemistry syllabus Rate of a reaction
Title: Understanding and Analyzing Reaction Rates: A Comprehensive White Paper
Abstract:
This white paper provides an in-depth exploration of the concept of reaction rates and their significance in chemical kinetics. It aims to enhance the understanding of scientists, researchers, and students in the field of chemistry. The paper discusses the fundamental principles of rate of a reaction, explores the factors influencing reaction rates, presents experimental methods for rate determination, and examines real-life applications. By delving into the intricacies of reaction rates, this white paper offers valuable insights and practical knowledge to advance the field of chemical kinetics.
Introduction:
1.1 Overview of Reaction Rates
1.2 Importance of Studying Reaction Rates
1.3 Significance in Various Fields
Principles of Reaction Rates:
2.1 Definition and Measurement of Reaction Rates
2.2 Rate Laws and Rate Expressions
2.3 Reaction Orders and Rate Constants
2.4 Rate-Determining Step and Transition State Theory
2.5 Collision Theory and Reaction Mechanisms
Factors Affecting Reaction Rates:
3.1 Concentration and Rate of Reaction
3.2 Temperature and Rate of Reaction
3.3 Catalysts and Reaction Rates
3.4 Surface Area and Reaction Rates
3.5 Pressure (for Gaseous Reactions) and Reaction Rates
Experimental Techniques for Rate Determination:
4.1 Initial Rate Method
4.2 Continuous Monitoring Method
4.3 Clock Reactions
4.4 Spectroscopic Methods
4.5 Kinetic Modeling and Data Analysis
Applications of Reaction Rates:
5.1 Chemical Engineering and Industrial Processes
5.2 Pharmaceutical Industry and Drug Kinetics
5.3 Environmental Science and Reaction Rates in Nature
5.4 Material Science and Kinetic Studies
5.5 Biological Systems and Enzyme Kinetics
Advanced Topics in Reaction Rates:
6.1 Temperature Dependence and Activation Energy
6.2 Reaction Order Determination
6.3 Rate Equations for Complex Reactions
6.4 Catalytic Kinetics and Catalyst Design
6.5 Reaction Kinetics in Non-Ideal Conditions
Conclusion:
7.1 Recap of Key Concepts
7.2 Future Directions and Emerging Research Areas
References:
Comprehensive list of cited works, textbooks, and additional resources for further exploration.
This white paper aims to provide a comprehensive and detailed analysis of the topic of reaction rates, catering to researchers, educators, and students seeking a deeper understanding of chemical kinetics. By covering the fundamental principles, experimental techniques, and practical applications, this white paper serves as a valuable resource for advancing the field of reaction rate studies.
