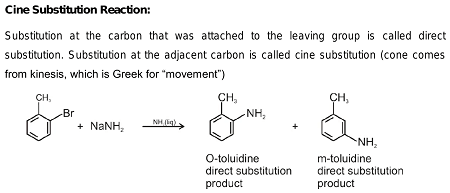
Haloarenes, which are organic compounds containing at least one halogen atom (fluorine, chlorine, bromine, or iodine) attached to an aromatic ring, can undergo cine substitution reactions.
Cine substitution reactions involve the substitution of a halogen atom on a benzene ring by a nucleophile, such as an amine, hydroxide ion, or cyanide ion, at the ortho or para position relative to the halogen atom. These reactions are also known as electrophilic aromatic substitution reactions because the halogen atom is an electron-withdrawing group that makes the ring more electrophilic and susceptible to attack by nucleophiles.
The mechanism of cine substitution involves the formation of a Wheland intermediate, which is a cyclic, highly reactive intermediate that is stabilized by resonance. The nucleophile attacks the Wheland intermediate, resulting in the substitution of the halogen atom.
Cine substitution reactions are useful in organic synthesis for the preparation of a variety of functionalized aromatic compounds, including pharmaceuticals and agrochemicals. However, the use of halogens in organic compounds can also have environmental and health implications, and efforts are being made to reduce their use and find alternative methods for these reactions.
What is Required Haloarenes Cine substitution
For a cine substitution reaction to occur in haloarenes, there are several requirements:
- Presence of a halogen atom: The haloarene must contain at least one halogen atom (fluorine, chlorine, bromine, or iodine) attached to an aromatic ring.
- Electron-withdrawing group effect: The halogen atom is an electron-withdrawing group, which makes the aromatic ring more electrophilic and susceptible to attack by nucleophiles.
- Nucleophile: A nucleophile must be present to attack the Wheland intermediate formed during the reaction. Common nucleophiles used in cine substitution reactions include amines, hydroxide ion, and cyanide ion.
- Conditions: The reaction conditions must be appropriate for the specific nucleophile used. For example, the reaction may require a certain temperature, solvent, or catalyst.
Overall, the requirements for a successful cine substitution reaction in haloarenes involve the presence of a halogen atom and an electron-withdrawing group effect, along with a suitable nucleophile and reaction conditions.
When is Required Haloarenes Cine substitution
Cine substitution reactions in haloarenes are used in organic synthesis for the preparation of a variety of functionalized aromatic compounds, including pharmaceuticals and agrochemicals.
Some specific applications of cine substitution reactions in haloarenes include:
- Preparation of substituted anilines: Anilines are a class of compounds used in the manufacture of dyes, drugs, and other chemicals. Cine substitution reactions can be used to prepare substituted anilines, which are important intermediates in the synthesis of pharmaceuticals and agrochemicals.
- Preparation of substituted phenols: Phenols are aromatic compounds containing a hydroxyl group (-OH) attached to the benzene ring. Cine substitution reactions can be used to prepare substituted phenols, which are important in the synthesis of pharmaceuticals and fragrances.
- Synthesis of heterocyclic compounds: Heterocyclic compounds are cyclic organic compounds containing at least one heteroatom (such as nitrogen, oxygen, or sulfur) in the ring. Cine substitution reactions can be used to prepare substituted heterocyclic compounds, which are important in the synthesis of pharmaceuticals and agrochemicals.
In summary, cine substitution reactions in haloarenes are required for the preparation of a wide range of important compounds in organic synthesis, including anilines, phenols, and heterocyclic compounds.
Where is Required Haloarenes Cine substitution
Cine substitution reactions in haloarenes can be used in various fields, such as pharmaceuticals, agrochemicals, and materials science. Here are some examples of where cine substitution reactions in haloarenes are required:
- Pharmaceuticals: Cine substitution reactions in haloarenes are used to prepare various pharmaceuticals, such as antihistamines, antidepressants, and antipsychotics. For example, the antidepressant drug fluoxetine (Prozac) is prepared by a cine substitution reaction of a halogenated aromatic compound.
- Agrochemicals: Cine substitution reactions in haloarenes are used to prepare various agrochemicals, such as herbicides, fungicides, and insecticides. For example, the herbicide glyphosate is prepared by a cine substitution reaction of a halogenated aromatic compound.
- Materials science: Cine substitution reactions in haloarenes are used to prepare various materials, such as liquid crystals, dyes, and polymers. For example, the liquid crystal material p-alkoxybenzoic acid is prepared by a cine substitution reaction of a halogenated aromatic compound.
Overall, cine substitution reactions in haloarenes are required in various fields where the preparation of functionalized aromatic compounds is necessary. These reactions play a crucial role in the development of new pharmaceuticals, agrochemicals, and materials with important applications in industry and research.
How is Required Haloarenes Cine substitution
Cine substitution reactions in haloarenes can be carried out using various methods, depending on the specific nucleophile and reaction conditions. Here is a general overview of the reaction mechanism and some common methods used for cine substitution reactions in haloarenes:
- Reaction mechanism: The reaction mechanism for cine substitution reactions in haloarenes involves the following steps:
- Electrophilic attack: The halogen atom on the haloarene is attacked by an electrophile (such as a Lewis acid) to form a Wheland intermediate, which is a highly reactive intermediate stabilized by resonance.
- Nucleophilic attack: The Wheland intermediate is attacked by a nucleophile (such as an amine, hydroxide ion, or cyanide ion) at the ortho or para position relative to the halogen atom.
- Deprotonation: Proton transfer occurs, and the final product is formed after the elimination of a hydrogen halide.
- Common methods: Some common methods for cine substitution reactions in haloarenes include:
- Sandmeyer reaction: The Sandmeyer reaction involves the conversion of a halogenated aromatic compound to an aryl diazonium salt, which can then undergo nucleophilic substitution at the ortho or para position.
- Balz-Schiemann reaction: The Balz-Schiemann reaction involves the conversion of a halogenated aromatic compound to a diazonium fluoroborate salt, which can then undergo nucleophilic substitution at the ortho or para position.
- Ullmann reaction: The Ullmann reaction involves the copper-mediated coupling of a halogenated aromatic compound with an amine, which undergoes nucleophilic substitution at the ortho or para position.
Overall, the choice of method for cine substitution reactions in haloarenes depends on the specific nucleophile and reaction conditions, as well as the desired product.
Production of Haloarenes Cine substitution
The production of haloarenes through cine substitution reactions typically involves the following steps:
- Halogenation of the starting arene: The starting arene is halogenated using a halogenating agent, such as chlorine or bromine. The halogenation can be carried out under different conditions depending on the reactivity of the arene and the desired degree of halogenation.
- Preparation of the nucleophile: The nucleophile used for the cine substitution reaction is prepared or obtained in a separate step. The nucleophile can be an amine, hydroxide ion, cyanide ion, or other suitable nucleophile, depending on the desired product.
- Cine substitution reaction: The halogenated arene is subjected to a cine substitution reaction with the prepared nucleophile. The reaction conditions depend on the specific nucleophile and the desired product. The reaction is typically carried out under basic or acidic conditions, and the temperature and reaction time are adjusted based on the reactivity of the arene and the nucleophile.
- Purification and isolation of the product: The product of the cine substitution reaction is purified and isolated using various techniques, such as distillation, recrystallization, or chromatography.
Overall, the production of haloarenes through cine substitution reactions requires careful control of reaction conditions to obtain high yields and selectivity. Various methods can be used to optimize the reaction conditions and to obtain the desired product.
Case Study on Haloarenes Cine substitution
One example of the use of haloarenes cine substitution is in the synthesis of the antidepressant drug fluoxetine (Prozac). Fluoxetine belongs to a class of drugs known as selective serotonin reuptake inhibitors (SSRIs), which are used to treat depression, anxiety, and other psychiatric disorders.
The synthesis of fluoxetine involves a number of steps, including the cine substitution of a halogenated arene. Here is a brief overview of the synthesis:
- Halogenation of starting arene: The starting arene, 4-chlorobenzotrifluoride, is halogenated with chlorine gas and iron catalyst to produce 4-chlorobenzotrifluoride chloride.
- Preparation of the nucleophile: An amine nucleophile, N-methyl-3-phenylpropylamine, is prepared through a multi-step synthesis.
- Cine substitution reaction: The 4-chlorobenzotrifluoride chloride is subjected to a cine substitution reaction with the prepared amine nucleophile under basic conditions. The reaction is carried out at elevated temperature for several hours, leading to the formation of 3-(p-trifluoromethylphenoxy)-N-methyl-3-phenylpropylamine, which is a key intermediate in the synthesis of fluoxetine.
- Further synthetic steps: The intermediate product is subjected to further synthetic steps, including protection and deprotection of functional groups, cyclization, and reduction, to yield the final product fluoxetine.
Overall, the cine substitution reaction in haloarenes plays a crucial role in the synthesis of fluoxetine and many other pharmaceuticals, agrochemicals, and materials. The careful control of reaction conditions and the choice of appropriate nucleophiles are essential for obtaining high yields and selectivity in the reaction.
White paper on Haloarenes Cine substitution
Introduction:
Haloarenes are compounds that contain a halogen atom (e.g., chlorine, bromine, or iodine) attached to an aromatic ring. These compounds are widely used in organic synthesis, medicinal chemistry, and material science due to their unique reactivity and physical properties. One important reaction that can be carried out in haloarenes is the cine substitution reaction, which involves the nucleophilic substitution of a halogen atom at the ortho or para position relative to the halogen. This white paper provides an overview of the mechanism, methods, and applications of cine substitution in haloarenes.
Mechanism:
The mechanism of cine substitution in haloarenes involves the following steps:
- Halogenation: The starting haloarene is halogenated using a halogenating agent (e.g., chlorine or bromine) to form a halogenated arene.
- Electrophilic attack: The halogenated arene is attacked by an electrophile (e.g., a Lewis acid) to form a highly reactive Wheland intermediate, which is stabilized by resonance.
- Nucleophilic attack: The Wheland intermediate is attacked by a nucleophile (e.g., an amine, hydroxide ion, or cyanide ion) at the ortho or para position relative to the halogen.
- Deprotonation: Proton transfer occurs, and the final product is formed after the elimination of a hydrogen halide.
Methods:
Several methods can be used to carry out cine substitution in haloarenes, including:
- Sandmeyer reaction: This reaction involves the conversion of a halogenated aromatic compound to an aryl diazonium salt, which can then undergo nucleophilic substitution at the ortho or para position.
- Balz-Schiemann reaction: This reaction involves the conversion of a halogenated aromatic compound to a diazonium fluoroborate salt, which can then undergo nucleophilic substitution at the ortho or para position.
- Ullmann reaction: This reaction involves the copper-mediated coupling of a halogenated aromatic compound with an amine, which undergoes nucleophilic substitution at the ortho or para position.
Applications:
Cine substitution in haloarenes has numerous applications in organic synthesis, medicinal chemistry, and material science. Some examples of its applications include:
- Synthesis of pharmaceuticals: Cine substitution is used in the synthesis of various pharmaceuticals, such as the antidepressant drug fluoxetine (Prozac) and the anti-inflammatory drug ibuprofen.
- Agrochemicals: Cine substitution is used in the synthesis of agrochemicals, such as the herbicide 2,4-dichlorophenoxyacetic acid (2,4-D).
- Materials science: Cine substitution is used in the synthesis of materials with specific properties, such as liquid crystals and conducting polymers.
Conclusion:
Cine substitution in haloarenes is a versatile reaction that has numerous applications in organic synthesis, medicinal chemistry, and material science. The careful choice of nucleophile and reaction conditions is essential for obtaining high yields and selectivity in the reaction. With the development of new methods and techniques, cine substitution in haloarenes is expected to continue to play an important role in the discovery and synthesis of new compounds and materials.
