
A colloid is a mixture in which one substance is dispersed in another substance, but not dissolved. The particles in a colloid are larger than molecules but smaller than the particles in a suspension. The properties of colloids are different from those of solutions or suspensions, and they have unique characteristics that make them useful in a variety of applications.
Types of colloids:
There are several types of colloids, including:
- Sol: A sol is a colloid in which solid particles are dispersed in a liquid. Examples include paint and ink.
- Gel: A gel is a colloid in which a liquid is dispersed in a solid. Examples include jelly and toothpaste.
- Emulsion: An emulsion is a colloid in which two immiscible liquids are dispersed in each other. Examples include milk and mayonnaise.
- Foam: A foam is a colloid in which a gas is dispersed in a liquid or solid. Examples include whipped cream and soap bubbles.
Methods of preparation:
There are several methods for preparing colloids, including:
- Mechanical dispersion: This involves grinding, milling, or shearing the particles to reduce their size and disperse them in the medium.
- Chemical methods: This involves the addition of a chemical agent to the medium to promote dispersion, such as a surfactant or emulsifying agent.
- Electrolytic dispersion: This involves the use of an electric field to disperse the particles in the medium.
General properties:
Colloids have several unique properties, including:
- Tyndall effect: This is the scattering of light by the particles in a colloid, which makes the colloid appear cloudy or milky.
- Brownian motion: The particles in a colloid are in constant motion due to collisions with molecules in the medium.
- Stability: Colloids can be stable for long periods of time due to repulsion between the particles, which prevents them from aggregating or settling out.
- Particle size: The particles in a colloid are typically between 1 nanometer and 1 micrometer in size.
- Surface area: Colloids have a high surface area-to-volume ratio, which makes them useful for catalysis and other chemical reactions.
Overall, colloids have a wide range of applications in industry, medicine, and everyday life, and their unique properties make them a valuable tool in many fields.
Colloid
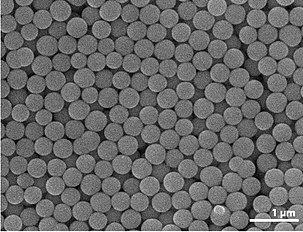
A colloid is a blend where one substance comprising of infinitesimally scattered insoluble particles is suspended all through another substance. A few definitions determine that the particles should be scattered in a fluid, while others stretch out the definition to incorporate substances like vapor sprayers and gels. The term colloidal suspension alludes unambiguously to the general combination (albeit a smaller feeling of the word suspension is recognized from colloids by bigger molecule size). A colloid has a scattered stage (the suspended particles) and a constant stage (the mechanism of suspension). The scattered stage particles have a measurement of roughly 1 nanometre to 1 micrometer.
A few colloids are clear a result of the Tyndall impact, which is the dissipating of light by particles in the colloid. Different colloids might be hazy or have a slight tone.
Colloidal suspensions are the subject of connection point and colloid science. This field of study started in 1845 by Francesco Selmi and extended by Michael Faraday and Thomas Graham, who begat the term colloid in 1861.
Colloid 1

A colloid is a combination of one substance spread out uniformly inside another substance. They can be in two unique stages or conditions of issue.
One substance is the scattering medium, like water or gas. The other is the scattered medium, once in a while called the ‘inner stage’. This is generally small strong particles. In any case, on the off chance that the scattering medium is a gas, the inside stage can be either little particles or small drops of a fluid.
Definition: A colloid is a substance minutely scattered uniformly all through another substance. The scattered stage particles have a width between around 5 and 200 nanometers.
Models:
Milk is an emulsion, which is a colloid where the two players are fluids.
Shaving cream and whipped cream are colloids of gas inside a fluid.
Gels, like agar, jam or even opals, are colloids of fluids inside solids.
Styrofoam and pumice are gases inside solids.
The red shade of cranberry glass is brought about by the impression of light off gold particles in the glass. The glass is a colloid of strong particles inside the strong glass.
Mud is normally viewed as a suspension on the grounds that the soil will settle, yet it could be a fractional colloid, especially with exceptionally fine-grained earth.
Haze is a perplexing blend wherein air has both water drops and strong particles. It very well might be colloidal in parts.
Hydrocolloids
Hydrocolloids depict specific synthetic substances (for the most part polysaccharides and proteins) that are colloidally dispersible in water. Consequently turning out to be actually “dissolvable” they change the rheology of water by raising the consistency and additionally prompting gelation. They might give other intuitive impacts different synthetic substances, at times synergistic, in others hostile. Utilizing these characteristics hydrocolloids are extremely helpful synthetic compounds since in numerous areas of innovation from food varieties through drugs, individual consideration and modern applications, they can give adjustment, destabilization and detachment, gelation, stream control, crystallization control and various different impacts. Aside from utilizations of the solvent structures a portion of the hydrocolloids have extra valuable usefulness in a dry structure on the off chance that after solubilization they have the water taken out – as in the development of movies for breath strips or hotdog housings or for sure, twisted dressing filaments, some being more viable with skin than others. There are various sorts of hydrocolloids each with contrasts in structure capability and utility that by and large are the most appropriate to specific application regions in the control of rheology and the actual alteration of structure and surface. A few hydrocolloids like starch and casein are helpful food varieties as well as rheology modifiers, others have restricted nutritive worth, typically giving a wellspring of fiber.
The term hydrocolloids likewise alludes to a sort of dressing intended to secure dampness in the skin and help the normal recuperating cycle of skin to diminish scarring, tingling and irritation.
Colloid compared with solution
A colloid has a scattered stage and a ceaseless stage, while in an answer, the solute and dissolvable comprise just a single stage. A solute in an answer are individual particles or particles, while colloidal particles are greater. For instance, in an answer of salt in water, the sodium chloride (NaCl) gem breaks down, and the Na+ and Cl− particles are encircled by water atoms. In any case, in a colloid, for example, milk, the colloidal particles are globules of fat, as opposed to individual fat particles. Since colloid is numerous stages, it has altogether different properties contrasted with completely blended, nonstop arrangement.
Case Study on Colloids types, methods of preparation and general properties
Sure, I can provide a brief case study on colloids to illustrate their types, methods of preparation, and general properties.
Case Study: Milk as a Colloid
Milk is a commonly consumed colloid that consists of dispersed fat globules and proteins in an aqueous solution. Let’s explore how milk fits into the types, methods of preparation, and general properties of colloids.
Types of Colloids in Milk:
Milk is an example of an emulsion, which is a type of colloid in which two immiscible liquids are dispersed in each other. In the case of milk, the immiscible liquids are fat and water.
Methods of Preparation of Milk:
Milk is produced through a mechanical dispersion method. The fat globules are mechanically dispersed in the aqueous solution of milk during the process of milking. After milking, the milk is pasteurized to kill any harmful bacteria.
General Properties of Milk:
- Milk has a high surface area-to-volume ratio, which allows it to easily interact with other substances in chemical reactions.
- Milk exhibits Brownian motion, which is a result of the dispersed fat globules moving randomly due to collisions with molecules in the aqueous solution.
- Milk exhibits the Tyndall effect, which is seen as the milk appears cloudy due to the scattering of light by the fat globules in the aqueous solution.
- Milk is stable for a period of time due to repulsion between the fat globules, which prevents them from aggregating and settling out of the aqueous solution.
Conclusion:
Milk is an example of a colloid, specifically an emulsion. It is prepared through a mechanical dispersion method and exhibits properties such as a high surface area-to-volume ratio, Brownian motion, and the Tyndall effect. These properties make milk useful in many applications, including cooking, baking, and in the production of various dairy products.
White paper on Colloids types, methods of preparation and general properties
Introduction:
Colloids are a type of mixture in which one substance is dispersed evenly throughout another substance, but not dissolved. The size of the dispersed particles in a colloid is larger than those in a solution, but smaller than those in a suspension. Colloids have a wide range of applications in various fields, including medicine, food, and industry. In this white paper, we will discuss the types, methods of preparation, and general properties of colloids.
Types of Colloids:
There are several types of colloids, including:
- Sol: A colloid in which solid particles are dispersed in a liquid. Examples include blood, ink, and paint.
- Gel: A colloid in which a liquid is dispersed in a solid. Examples include Jell-O and rubber.
- Emulsion: A colloid in which two immiscible liquids are dispersed in each other. Examples include milk and salad dressing.
- Foam: A colloid in which a gas is dispersed in a liquid or solid. Examples include whipped cream and shaving cream.
Methods of Preparation:
There are various methods of preparing colloids, including:
- Mechanical dispersion: This involves grinding, milling, or shearing the particles to reduce their size and disperse them in the medium. Examples include the use of a ball mill to grind pigment particles for ink and paint.
- Chemical methods: This involves the addition of a chemical agent to the medium to promote dispersion, such as a surfactant or emulsifying agent. Examples include the use of lecithin in chocolate to prevent the separation of cocoa solids and cocoa butter.
- Electrolytic dispersion: This involves the use of an electric field to disperse the particles in the medium. Examples include the use of electrophoresis to separate DNA fragments in molecular biology.
General Properties:
Colloids exhibit several general properties, including:
- Brownian motion: Colloidal particles exhibit Brownian motion, in which they move randomly due to collisions with molecules in the medium.
- Surface area-to-volume ratio: Colloids have a high surface area-to-volume ratio, which makes them useful for catalysis and other chemical reactions.
- Tyndall effect: Colloids exhibit the Tyndall effect, in which the dispersed particles scatter light and cause the colloid to appear cloudy.
- Stability: Colloids can be stable for long periods of time due to repulsion between the particles, which prevents them from aggregating or settling out.
Conclusion:
In conclusion, colloids are a type of mixture that have several applications in various fields. They can be prepared using different methods, such as mechanical dispersion, chemical methods, and electrolytic dispersion. Colloids exhibit several general properties, such as Brownian motion, a high surface area-to-volume ratio, the Tyndall effect, and stability. Understanding the types, methods of preparation, and general properties of colloids can lead to their more effective use in a variety of applications.
