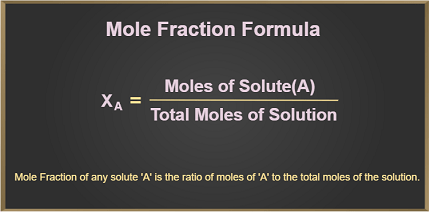
Concentration can be expressed in terms of mole fraction, which is defined as the ratio of the number of moles of a particular substance to the total number of moles in the mixture.
The mole fraction of a substance A in a mixture of n substances can be calculated using the following formula:
Mole fraction of A = Number of moles of A / Total number of moles in the mixture
The mole fraction of a substance always lies between 0 and 1. The sum of the mole fractions of all the substances in a mixture is always equal to 1.
Mole fraction is a useful way to express the concentration of a substance in a mixture, particularly in solutions. It is independent of the physical state of the substance and is not affected by changes in temperature or pressure.
What is Required Concentration in terms of mole fraction
Required concentration in terms of mole fraction refers to the desired mole fraction of a particular substance in a mixture. This can be calculated based on the desired overall concentration of the mixture and the mole fraction of other substances present in the mixture.
For example, suppose we have a mixture of two substances, A and B, with mole fractions of xA and xB, respectively. If we want to achieve a total concentration of C moles per liter and we want the mole fraction of substance A to be yA, we can calculate the required mole fraction of substance B as follows:
Total number of moles in the mixture = xA + xB
Number of moles of substance A = yA x C
Number of moles of substance B = C – (yA x C)
Mole fraction of substance B = (Number of moles of substance B) / (Total number of moles in the mixture) = [(C – (yA x C)) / C] / (1 – yA)
This gives us the required mole fraction of substance B that we need to achieve the desired overall concentration of the mixture with the desired mole fraction of substance A.
When is Required Concentration in terms of mole fraction
The concept of “required concentration in terms of mole fraction” is used whenever a chemist or researcher needs to calculate the amount of a particular substance that needs to be added to a mixture in order to achieve a desired concentration and mole fraction. This could occur in a variety of situations, such as:
- When preparing a solution: A chemist may need to prepare a solution of a particular concentration and mole fraction, and will use this concept to calculate the amount of solute needed to achieve the desired properties.
- When analyzing a mixture: A researcher may need to determine the concentration and mole fraction of a particular substance in a mixture, and will use this concept to calculate the expected amounts of different substances in the mixture.
- When optimizing a process: In industrial settings, it may be important to achieve a certain concentration and mole fraction of a substance in order to optimize a particular process or reaction. In this case, chemists will use this concept to determine the appropriate amounts of different substances to add to the mixture.
In summary, the concept of required concentration in terms of mole fraction is used whenever precise control over the composition of a mixture is required, whether for research or industrial purposes.
Where is Required Concentration in terms of mole fraction
The concept of “required concentration in terms of mole fraction” can be applied to any mixture of substances, whether it is a solution, a gas mixture, or a solid mixture. The location of this concept is not specific to any physical location, but rather is a mathematical concept that can be used in various contexts in chemistry, including in laboratories, research facilities, and industrial settings.
For example, a chemist may use this concept in a laboratory to prepare a solution with a specific concentration and mole fraction of a particular solute. Similarly, an engineer in a chemical plant may use this concept to optimize the composition of a gas mixture used in a chemical reaction.
In short, the concept of required concentration in terms of mole fraction is a fundamental concept in chemistry that is applicable to a wide range of settings and situations.
How is Required Concentration in terms of mole fraction
The “required concentration in terms of mole fraction” can be calculated using the following formula:
Mole fraction of substance A = (Number of moles of substance A) / (Total number of moles in the mixture)
To calculate the required concentration in terms of mole fraction for a given mixture, we need to know the desired mole fraction of the substance in question, as well as the mole fractions of all the other substances in the mixture. The total number of moles in the mixture can be calculated by summing the number of moles of each substance present.
Once we have this information, we can use the formula above to calculate the number of moles of the substance required to achieve the desired mole fraction. This can be done using algebra to solve for the unknown quantity (the number of moles of substance A). Once we have the number of moles of substance A, we can then calculate the amount of the substance needed to achieve this quantity, based on its molar mass.
In summary, the required concentration in terms of mole fraction can be calculated using basic mathematical concepts and information about the mole fractions of different substances in a mixture. This calculation allows chemists and researchers to precisely control the composition of a mixture for various purposes, including preparing solutions, analyzing mixtures, and optimizing chemical processes.
Production of Concentration in terms of mole fraction
The production of concentration in terms of mole fraction involves determining the number of moles of a particular substance present in a mixture relative to the total number of moles in the mixture. This can be achieved through various methods depending on the type of mixture being analyzed.
For a solution, the concentration in terms of mole fraction can be determined by measuring the mass or volume of the solute and solvent, converting it to moles using the molar mass or density of the substance, and then calculating the mole fraction using the formula:
x = (moles of solute) / (moles of solute + moles of solvent)
For a gas mixture, the concentration in terms of mole fraction can be determined by measuring the pressure, volume, and temperature of the gas and using the ideal gas law to calculate the number of moles of each component in the mixture. The mole fraction can then be calculated using the formula:
x = (moles of component) / (total moles in the mixture)
In the case of a solid mixture, the concentration in terms of mole fraction can be determined by analyzing the mass or volume of each component and converting it to moles using the molar mass of the substance. The mole fraction can then be calculated using the formula:
x = (moles of component) / (total moles in the mixture)
Overall, the production of concentration in terms of mole fraction involves accurate measurement of the components present in a mixture and the use of mathematical formulas to calculate the number of moles of each component and the total number of moles in the mixture. By controlling the mole fraction of different substances in a mixture, researchers can achieve precise control over the properties of the mixture for various applications.
Case Study on Concentration in terms of mole fraction
Let’s consider the following case study as an example of how concentration in terms of mole fraction can be applied in practice:
Case Study: Preparing a Solution of Ethanol in Water
A chemist needs to prepare a solution of ethanol in water with a mole fraction of 0.2. The desired total concentration of the solution is 0.5 moles per liter. How much ethanol (in grams) should the chemist add to 1 liter of water to achieve the desired mole fraction?
Solution:
Step 1: Calculate the mole fraction of water in the solution.
The mole fraction of water (xW) can be calculated using the following formula:
xW = (moles of water) / (total moles in the solution)
Since we want the mole fraction of ethanol to be 0.2, the mole fraction of water can be calculated as:
xW = 1 – 0.2 = 0.8
Step 2: Calculate the number of moles of water in the solution.
The total concentration of the solution is given as 0.5 moles per liter, so the number of moles of water (nW) in 1 liter of the solution can be calculated as:
nW = xW * concentration = 0.8 * 0.5 = 0.4 moles
Step 3: Calculate the number of moles of ethanol in the solution.
Since we want the mole fraction of ethanol to be 0.2, the number of moles of ethanol (nE) in 1 liter of the solution can be calculated as:
nE = xE * concentration = 0.2 * 0.5 = 0.1 moles
Step 4: Calculate the mass of ethanol needed to prepare the solution.
The molar mass of ethanol is 46.07 g/mol. Therefore, the mass of ethanol (mE) needed to prepare the solution can be calculated as:
mE = nE * molar mass = 0.1 * 46.07 = 4.607 g
Therefore, the chemist needs to add 4.607 grams of ethanol to 1 liter of water to prepare a solution with a mole fraction of ethanol of 0.2 and a total concentration of 0.5 moles per liter.
This case study demonstrates how concentration in terms of mole fraction can be used to calculate the amount of a substance needed to prepare a solution with a specific composition. By controlling the mole fraction of different substances in a mixture, chemists and researchers can achieve precise control over the properties of the mixture for various purposes.
White paper on Concentration in terms of mole fraction
Introduction
Concentration in terms of mole fraction is a fundamental concept in chemistry that describes the amount of a particular substance present in a mixture. Mole fraction is a way of expressing the composition of a mixture in terms of the number of moles of each component relative to the total number of moles in the mixture. This white paper provides an overview of the concept of concentration in terms of mole fraction, its calculation, and its applications in various fields of chemistry.
Mole Fraction
The mole fraction of a substance in a mixture is defined as the ratio of the number of moles of the substance to the total number of moles in the mixture. It is denoted by the symbol “x” and is expressed as a decimal or a fraction. The mole fraction of a substance is always between zero and one.
The mole fraction of a substance can be calculated using the following formula:
x = (moles of substance) / (total moles in the mixture)
For example, if a solution contains 2 moles of solute and 3 moles of solvent, the mole fraction of the solute can be calculated as:
x solute = (2 moles) / (2 moles + 3 moles) = 0.4
Concentration in terms of Mole Fraction
Concentration in terms of mole fraction is a way of expressing the amount of a particular substance in a mixture relative to the total amount of all the substances in the mixture. It is useful for describing the composition of solutions, gas mixtures, and solid mixtures. In a solution, the concentration in terms of mole fraction can be used to describe the amount of solute dissolved in a given amount of solvent.
The concentration in terms of mole fraction is calculated using the following formula:
Concentration in terms of mole fraction = (moles of the substance) / (total moles in the mixture)
Applications
Concentration in terms of mole fraction is a useful concept in many areas of chemistry. In analytical chemistry, it is used to determine the composition of a mixture by measuring the mole fraction of each component. In physical chemistry, it is used to study the properties of solutions, including vapor pressure, boiling point, and osmotic pressure.
In industrial chemistry, concentration in terms of mole fraction is used to optimize the composition of gas mixtures used in chemical reactions. For example, the mole fraction of reactants in a gas mixture can be adjusted to maximize the yield of a desired product. In addition, concentration in terms of mole fraction is used to prepare solutions with specific concentrations and compositions for various applications, including medicine, biology, and environmental science.
Conclusion
Concentration in terms of mole fraction is a fundamental concept in chemistry that describes the amount of a particular substance in a mixture relative to the total amount of all the substances in the mixture. It is useful for describing the composition of solutions, gas mixtures, and solid mixtures. The mole fraction of a substance can be calculated using the number of moles of the substance and the total number of moles in the mixture. By controlling the mole fraction of different substances in a mixture, chemists and researchers can achieve precise control over the properties of the mixture for various purposes.