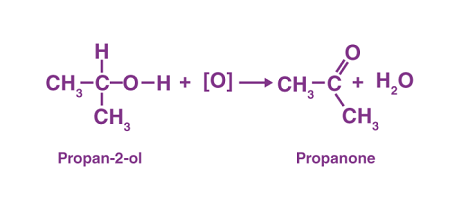
Alcohols can be converted into aldehydes through oxidation reactions. One commonly used method is the use of an oxidizing agent such as chromic acid (H2CrO4), pyridinium chlorochromate (PCC), or Jones reagent (a solution of chromic acid and sulfuric acid). The specific oxidizing agent and conditions used will depend on the alcohol being converted and the desired product.
For example, primary alcohols can be oxidized to aldehydes using PCC or the Jones reagent under mild conditions. The reaction typically takes place at room temperature and in the absence of water to prevent further oxidation to carboxylic acids. Secondary alcohols can also be converted into ketones through oxidation reactions using similar methods.
Overall, the oxidation of alcohols to aldehydes is an important synthetic transformation used in organic chemistry for the preparation of various compounds.
What is Required Conversion of alcohols into aldehydes
The conversion of alcohols into aldehydes typically requires an oxidizing agent and mild reaction conditions. The choice of oxidizing agent will depend on the specific alcohol being converted and the desired product.
One common oxidizing agent used for this conversion is pyridinium chlorochromate (PCC). PCC is a mild and selective oxidizing agent that is often used to convert primary alcohols to aldehydes. The reaction typically takes place at room temperature and in the absence of water to prevent further oxidation to carboxylic acids.
Another oxidizing agent used for this conversion is the Jones reagent, which is a mixture of chromic acid and sulfuric acid. The Jones reagent is a stronger oxidizing agent compared to PCC and can be used to oxidize both primary and secondary alcohols to their corresponding aldehydes.
In addition to the oxidizing agent, the reaction conditions are also important for the successful conversion of alcohols to aldehydes. The reaction must be carried out in the absence of water and at a controlled temperature to prevent further oxidation to carboxylic acids or over-oxidation to ketones. The use of a catalyst or a solvent can also improve the efficiency of the reaction.
Overall, the conversion of alcohols to aldehydes requires careful selection of the oxidizing agent and reaction conditions to achieve the desired product with high selectivity and efficiency.
When is Required Conversion of alcohols into aldehydes
The conversion of alcohols into aldehydes is an important reaction in organic chemistry and is used for the synthesis of various compounds. Some of the common applications of this conversion include:
- Synthesis of fragrances and flavors: Aldehydes are important building blocks in the synthesis of fragrances and flavors. For example, the conversion of benzyl alcohol to benzaldehyde is a key step in the synthesis of almond fragrance.
- Synthesis of pharmaceuticals: Aldehydes are used as key intermediates in the synthesis of various pharmaceutical compounds. For example, the conversion of benzyl alcohol to benzaldehyde followed by a reduction step can be used to synthesize benzyl alcohol derivatives that have antihistamine or antitussive properties.
- Polymerization reactions: Aldehydes can undergo polymerization reactions to form various types of polymers. For example, formaldehyde can undergo polymerization to form polyoxymethylene, which is used as a thermoplastic in various applications.
- Organic synthesis: Aldehydes can be used as versatile building blocks in organic synthesis for the preparation of various compounds, such as alcohols, acids, and ketones.
Overall, the conversion of alcohols into aldehydes is an important reaction that finds wide applications in organic synthesis, pharmaceuticals, fragrances, and polymers.
Where is Required Conversion of alcohols into aldehydes
The conversion of alcohols into aldehydes can be carried out in various settings, including industrial, academic, and research laboratories.
In the industrial setting, this conversion can be used in the production of fragrances, flavors, and pharmaceuticals. For example, the conversion of benzyl alcohol to benzaldehyde is an important step in the production of almond flavoring and fragrance.
In academic and research laboratories, this conversion is used for the synthesis of various organic compounds and for studying the properties of aldehydes. The conversion can be carried out using various oxidizing agents and reaction conditions, and the resulting aldehydes can be used as building blocks for further synthesis.
Overall, the conversion of alcohols into aldehydes is an important reaction that is widely used in various settings for the production of various compounds and for the advancement of organic chemistry research.
How is Required Conversion of alcohols into aldehydes
The conversion of alcohols into aldehydes can be carried out through oxidation reactions using various oxidizing agents and reaction conditions.
One common oxidizing agent used for this conversion is pyridinium chlorochromate (PCC). The reaction typically takes place at room temperature and in the absence of water to prevent further oxidation to carboxylic acids. The reaction proceeds through a mechanism that involves the formation of a chromate ester intermediate, which is then hydrolyzed to yield the aldehyde product.
Another oxidizing agent that can be used for this conversion is the Jones reagent, which is a mixture of chromic acid and sulfuric acid. The Jones reagent is a stronger oxidizing agent compared to PCC and can be used to oxidize both primary and secondary alcohols to their corresponding aldehydes. The reaction proceeds through a mechanism that involves the formation of a chromate ester intermediate, which is then hydrolyzed to yield the aldehyde product.
In addition to these oxidizing agents, other reagents such as sodium dichromate and potassium permanganate can also be used for the conversion of alcohols to aldehydes.
The reaction conditions are also important for the successful conversion of alcohols to aldehydes. The reaction must be carried out in the absence of water and at a controlled temperature to prevent further oxidation to carboxylic acids or over-oxidation to ketones. The use of a catalyst or a solvent can also improve the efficiency of the reaction.
Overall, the conversion of alcohols to aldehydes involves the use of an oxidizing agent and careful control of reaction conditions to achieve the desired product with high selectivity and efficiency.
Nomenclature of Conversion of alcohols into aldehydes
The conversion of alcohols into aldehydes does not change the basic nomenclature of the starting alcohol or the resulting aldehyde.
In the IUPAC nomenclature system, alcohols are named by replacing the -e suffix of the corresponding alkane with -ol. For example, ethanol is the alcohol derived from ethane. Aldehydes are named by replacing the -e suffix of the corresponding alkane with -al. For example, formaldehyde is the aldehyde derived from methane.
The conversion of an alcohol to an aldehyde involves the oxidation of the -OH functional group to a -CHO functional group. However, the basic structure of the molecule remains the same, and thus the nomenclature of the molecule does not change.
For example, the conversion of ethanol to acetaldehyde involves the oxidation of the -OH group to a -CHO group:
CH3CH2OH (ethanol) + [O] → CH3CHO (acetaldehyde) + H2O
In this reaction, the basic structure of the molecule remains the same, and thus the nomenclature of the starting alcohol (ethanol) and the resulting aldehyde (acetaldehyde) does not change.
Overall, the nomenclature of the conversion of alcohols into aldehydes follows the basic rules of the IUPAC nomenclature system for naming alcohols and aldehydes.
Case Study on Conversion of alcohols into aldehydes
One case study for the conversion of alcohols into aldehydes involves the production of benzaldehyde, an important intermediate in the production of various fragrances, flavors, and pharmaceuticals. Benzaldehyde is commonly used as a flavoring agent for foods and beverages, and as a fragrance in perfumes and cosmetics.
The production of benzaldehyde from benzyl alcohol can be carried out using various oxidizing agents, including pyridinium chlorochromate (PCC), sodium dichromate, and potassium permanganate. In this case study, we will focus on the use of PCC for the conversion of benzyl alcohol to benzaldehyde.
The reaction can be carried out as follows:
- Dissolve benzyl alcohol in anhydrous dichloromethane (DCM) to form a solution.
- Add PCC to the solution and stir the mixture at room temperature for several hours.
- After the reaction is complete, the mixture is filtered to remove the PCC and the resulting solid byproducts.
- The organic layer is then extracted with water to remove any remaining PCC and byproducts.
- The organic layer is then dried with anhydrous magnesium sulfate and the solvent is evaporated to yield the crude benzaldehyde product.
- The crude product can be purified by distillation or recrystallization to yield pure benzaldehyde.
The mechanism for the reaction involves the oxidation of the -OH group in benzyl alcohol to a -CHO group, as shown below:
PhCH2OH + PCC → PhCHO + PCC-H2O
In this reaction, the PCC acts as the oxidizing agent and forms a chromate ester intermediate, which then undergoes hydrolysis to yield benzaldehyde and the PCC-H2O byproduct.
Overall, the conversion of benzyl alcohol to benzaldehyde using PCC is a useful method for the production of this important intermediate in the fragrance, flavor, and pharmaceutical industries.
White paper on Conversion of alcohols into aldehydes
Introduction:
The conversion of alcohols into aldehydes is an important transformation in organic chemistry. Aldehydes are versatile building blocks in the synthesis of various organic compounds, such as fragrances, flavors, pharmaceuticals, and agrochemicals. The conversion of alcohols to aldehydes is achieved by the oxidation of the -OH group in the alcohol to a -CHO group in the aldehyde. In this white paper, we will discuss the various methods for the conversion of alcohols into aldehydes and their applications.
Methods for Conversion of Alcohols to Aldehydes:
There are several methods for the conversion of alcohols to aldehydes, including oxidation with various oxidizing agents, dehydrogenation, and reductive deoxygenation.
Oxidation with Various Oxidizing Agents:
One of the most common methods for the conversion of alcohols to aldehydes is oxidation with various oxidizing agents, such as pyridinium chlorochromate (PCC), sodium dichromate, potassium permanganate, and others. These oxidizing agents are typically used in the presence of a solvent, such as dichloromethane or acetonitrile.
Dehydrogenation:
Another method for the conversion of alcohols to aldehydes is dehydrogenation, which involves the removal of two hydrogen atoms from the alcohol molecule. This can be achieved using various catalysts, such as copper chromite, copper oxide, or platinum. Dehydrogenation is typically carried out at high temperatures and pressures.
Reductive Deoxygenation:
Reductive deoxygenation is a method for the conversion of alcohols to aldehydes that involves the reduction of the -OH group in the alcohol to a -CH2 group in the aldehyde. This can be achieved using various reducing agents, such as lithium aluminum hydride, sodium borohydride, or hydrogen gas over a metal catalyst.
Applications:
The conversion of alcohols to aldehydes has various applications in the chemical industry, particularly in the synthesis of fragrances, flavors, and pharmaceuticals. For example, benzaldehyde is a commonly used flavoring agent and fragrance in the food and perfume industries. It is synthesized by the oxidation of benzyl alcohol using PCC or other oxidizing agents. Formaldehyde is another important aldehyde that is used in the production of various resins and polymers.
Conclusion:
In conclusion, the conversion of alcohols to aldehydes is an important transformation in organic chemistry with various applications in the chemical industry. There are several methods for the conversion of alcohols to aldehydes, including oxidation with various oxidizing agents, dehydrogenation, and reductive deoxygenation. These methods have been used in the synthesis of various fragrances, flavors, and pharmaceuticals. The choice of method depends on the specific reaction conditions and desired product.
