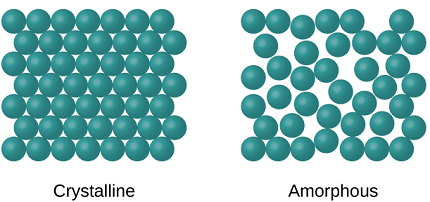
The crystalline state is a state of matter in which the constituent particles, such as atoms, molecules, or ions, are arranged in a highly ordered, repeating three-dimensional pattern called a crystal lattice. In this state, the particles are held together by strong intermolecular forces and exhibit a characteristic set of physical and chemical properties, such as high melting and boiling points, well-defined geometric shapes, and optical properties.
Crystals can be found in a variety of natural and synthetic materials, including metals, minerals, salts, and organic compounds. They can be grown from a solution, vapor, or melt using various techniques, such as crystal pulling, Bridgman-Stockbarger method, or Czochralski process. The study of crystals and their properties is known as crystallography, which is an important field in materials science, chemistry, and physics.
The crystalline state is one of the four fundamental states of matter, the others being the solid, liquid, and gas states. While solids and liquids can also exhibit ordered structures, the crystalline state is distinguished by its highly ordered and repetitive arrangement of particles.
Crystal


A precious stone or glasslike strong is a strong material whose constituents (like iotas, particles, or particles) are organized in an exceptionally requested minute construction, shaping a gem cross section that reaches out every which way. Also, plainly visible single precious stones are generally recognizable by their mathematical shape, comprising of level appearances with explicit, trademark directions. The logical investigation of precious stones and gem development is known as crystallography. The course of precious stone development through systems of gem development is called crystallization or cementing.
The word precious stone gets from the Antiquated Greek word κρύσταλλος (krustallos), meaning both “ice” and “rock gem”, from κρύος (kruos), “frigid cold, ice”.
Instances of enormous gems incorporate snowflakes, jewels, and table salt. Most inorganic solids are not precious stones but rather polycrystals, for example numerous minute gems intertwined into a solitary strong. Polycrystals incorporate most metals, rocks, pottery, and ice. A third class of solids is nebulous solids, where the iotas have no occasional design at all. Instances of nebulous solids incorporate glass, wax, and numerous plastics.
Regardless of the name, lead gem, precious stone glass, and related items are not precious stones, but instead kinds of glass, for example shapeless solids.
Precious stones, or glasslike solids, are in many cases utilized in pseudoscientific practices like gem treatment, and, alongside gemstones, are at times connected with spellwork in Wiccan convictions and related strict developments.
Crystal structure
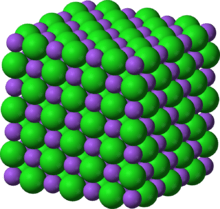
In crystallography, precious stone design is a depiction of the arranged game plan of iotas, particles, or atoms in a translucent material. Requested structures happen from the characteristic idea of the constituent particles to shape symmetric examples that recurrent along the foremost bearings of three-layered space in issue.
The littlest gathering of particles in the material that is this rehashing design is the unit cell of the construction. The unit cell totally mirrors the evenness and design of the whole precious stone, which is developed by dreary interpretation of the unit cell along its chief tomahawks. The interpretation vectors characterize the hubs of the Bravais cross section.
The lengths of the chief tomahawks, or edges, of the unit cell and the points between them are the cross section constants, additionally called grid boundaries or cell boundaries. The balance properties of the gem are depicted by the idea of room gatherings. All conceivable symmetric courses of action of particles in three-layered space might be portrayed by the 230 space gatherings.
The precious stone design and evenness assume a basic part in deciding numerous actual properties, for example, cleavage, electronic band structure, and optical straightforwardness.
Crystallography
Crystallography is the exploratory study of deciding the game plan of particles in translucent solids. Crystallography is a basic subject in the fields of materials science and strong state physical science (dense matter physical science). “Crystallography” is gotten from the Greek word κρύσταλλος (krystallos) “clear ice, rock-gem”, with its importance stretching out to all solids with some level of straightforwardness, and γράφειν (graphein) “to compose”. In July 2012, the Assembled Countries perceived the significance of the study of crystallography by announcing that 2014 would be the Global Year of Crystallography.
Prior to the improvement of X-beam diffraction crystallography (see underneath), the investigation of precious stones depended on actual estimations of their math utilizing a goniometer. This elaborate estimating the points of precious stone faces comparative with one another and to hypothetical reference tomahawks (crystallographic tomahawks), and laying out the evenness of the gem being referred to. The situation in 3D space of every gem face is plotted on a stereographic net, for example, a Wulff net or Lambert net. The shaft to each face is plotted on the net. Each point is marked with its Mill operator list. The last plot permits the evenness of the gem to be laid out.
Crystallographic strategies presently rely upon examination of the diffraction examples of an example designated by a light emission type. X-beams are generally regularly utilized; different bars utilized incorporate electrons or neutrons. Crystallographers frequently unequivocally express the sort of shaft utilized, as in the terms X-beam crystallography, neutron diffraction and electron diffraction. These three sorts of radiation cooperate with the example in various ways.
X-beams communicate with the spatial dispersion of electrons in the example.
Electrons are charged particles and in this way cooperate with the absolute charge appropriation of both the nuclear cores and the electrons of the example.
Neutrons are dissipated by the nuclear cores through major areas of strength for the powers, yet furthermore, the attractive snapshot of neutrons is non-zero. They are consequently likewise dissipated by attractive fields. At the point when neutrons are dispersed from hydrogen-containing materials, they produce diffraction designs with high clamor levels. Be that as it may, the material can some of the time be blessed to receive substitute deuterium for hydrogen. On account of these various types of connection, the three kinds of radiation are appropriate for various crystallographic studies.
Crystallization

Crystallization is the cycle by which strong structures, where the iotas or particles are profoundly coordinated into a design known as a gem. A few different ways by which gems structure are hastening from an answer, freezing, or all the more seldom statement straightforwardly from a gas. Qualities of the subsequent gem rely generally upon variables, for example, temperature, pneumatic stress, and on account of fluid precious stones, season of liquid vanishing.
Crystallization happens in two significant stages. The first is nucleation, the presence of a glasslike stage from either a supercooled fluid or a supersaturated dissolvable. The subsequent step is known as precious stone development, which is the expansion in the size of particles and prompts a gem state. A significant component of this step is that free particles structure layers at the gem’s surface and cabin themselves into open irregularities like pores, breaks, and so on.
Most of minerals and natural atoms take shape effectively, and the subsequent gems are by and large of good quality, for example without apparent imperfections. Be that as it may, bigger biochemical particles, similar to proteins, are frequently hard to take shape. The straightforwardness with which particles will solidify unequivocally relies upon the power of either nuclear powers (on account of mineral substances), intermolecular powers (natural and biochemical substances) or intramolecular powers (biochemical substances).
Crystallization is likewise a substance strong fluid detachment strategy, in which mass exchange of a solute from the fluid answer for an unadulterated strong glasslike stage happens. In compound designing, crystallization happens in a crystallizer. Crystallization is consequently connected with precipitation, albeit the outcome isn’t formless or confused, yet a precious stone.
Crystal system
In crystallography, a gem framework is a bunch of point gatherings (a gathering of mathematical balances with something like one fixed point). A grid framework is a bunch of Bravais cross sections. Space bunches are characterized into gem frameworks as indicated by their point gatherings, and into grid frameworks as per their Bravais cross sections. Precious stone frameworks that have space bunches doled out to a typical cross section framework are consolidated into a gem family.
The seven gem frameworks are triclinic, monoclinic, orthorhombic, tetragonal, three-sided, hexagonal, and cubic. Casually, two gems are in a similar precious stone framework on the off chance that they have comparative balances (but there are numerous exemptions).
Case Study on Crystalline state
Sure, I can provide a case study on the crystalline state. Here is an example:
Case Study: The Crystalline State of Diamond
Diamond is a well-known example of a material in the crystalline state. It is a form of carbon that is highly valued for its hardness, brilliance, and optical properties. Diamond is composed of carbon atoms that are arranged in a highly ordered, repeating pattern known as a diamond lattice.
The diamond lattice is a three-dimensional network of carbon atoms, with each atom bonded to four neighboring atoms in a tetrahedral arrangement. This gives diamond its characteristic geometric shape and makes it one of the hardest materials known to man. The interatomic bonds in diamond are very strong, which makes it difficult to break or deform the crystal lattice.
Diamond is found naturally in the earth’s crust, but it can also be synthesized through a variety of techniques, such as high-pressure high-temperature (HPHT) methods and chemical vapor deposition (CVD). The resulting diamonds may have slightly different properties depending on the synthesis method used.
The properties of diamond in the crystalline state are remarkable. It has a high melting point of over 3,000°C, is a good electrical insulator, and has a very high refractive index, which makes it highly prized in the jewelry industry. Its hardness and abrasion resistance also make it useful in cutting tools and other industrial applications.
In conclusion, diamond is a prime example of a material in the crystalline state. Its highly ordered diamond lattice structure and unique properties make it a valuable material in many different fields. The study of diamond and other crystalline materials is an important area of research in materials science, chemistry, and physics.
White paper on Crystalline state
Sure, here is a white paper on the crystalline state:
Introduction
The crystalline state is a fundamental concept in materials science, chemistry, and physics. It refers to the state of matter in which the constituent particles are arranged in a highly ordered, repeating pattern known as a crystal lattice. This state is characterized by a set of physical and chemical properties that are distinct from those of other states of matter, such as liquids and gases.
The purpose of this white paper is to provide an overview of the crystalline state, including its properties, applications, and current research trends.
Properties of the Crystalline State
The crystalline state is characterized by several unique properties that arise from the highly ordered arrangement of particles in the crystal lattice. Some of these properties include:
- Definite shape and volume: Crystals have a fixed shape and volume, which is determined by the arrangement of particles in the crystal lattice.
- Melting and boiling points: Crystals typically have high melting and boiling points due to the strong intermolecular forces between particles in the crystal lattice.
- Optical properties: Crystals can exhibit a range of optical properties, such as birefringence, polarization, and fluorescence, which make them useful in various applications.
- Electronic properties: The ordered structure of the crystal lattice can also give rise to unique electronic properties, such as band structure and conductivity, which have important applications in semiconductor technology.
Applications of the Crystalline State
The crystalline state has many important applications in various fields. Some of these applications include:
- Semiconductor technology: Crystalline materials are used extensively in the manufacture of electronic devices, such as transistors, integrated circuits, and solar cells.
- Optics: Crystals are used in various optical devices, such as lenses, prisms, and lasers.
- Jewelry: Crystals, such as diamonds, are highly valued for their optical and physical properties and are used in jewelry.
- Pharmaceuticals: Many drugs are crystalline compounds that are designed to have specific properties, such as solubility and bioavailability.
Current Research Trends in the Crystalline State
There are several active areas of research in the crystalline state. Some of these include:
- Novel materials: Researchers are developing new crystalline materials with unique properties and applications, such as materials for energy storage and conversion.
- Crystal engineering: The field of crystal engineering involves designing and synthesizing crystals with specific properties for various applications.
- Structural biology: Crystalline materials are used extensively in structural biology to determine the structure of biological molecules, such as proteins and nucleic acids.
- Quantum materials: Researchers are studying the electronic and magnetic properties of crystalline materials to develop new quantum technologies, such as quantum computers.
Conclusion
In conclusion, the crystalline state is a fundamental concept in materials science, chemistry, and physics. It has a range of unique properties that make it useful in various applications, from electronics to jewelry to pharmaceuticals. Current research trends in the crystalline state are focused on developing new materials with unique properties and understanding the electronic and magnetic properties of crystalline materials for quantum technologies.
