Cv and Cp are thermodynamic properties that describe the heat capacity of a substance at constant volume (Cv) and constant pressure (Cp), respectively.
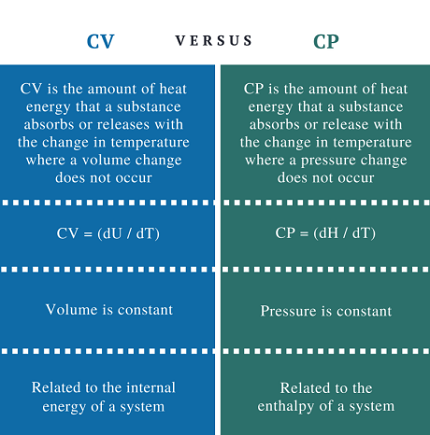
Cv is defined as the amount of heat required to raise the temperature of a substance by one degree Celsius at constant volume. It represents the ability of a substance to store heat energy without changing its volume.
Cp, on the other hand, is defined as the amount of heat required to raise the temperature of a substance by one degree Celsius at constant pressure. It represents the ability of a substance to store heat energy while allowing for expansion or contraction due to changes in pressure.
The difference between Cv and Cp is that Cp takes into account the work done by the substance as it expands or contracts under constant pressure. As a result, Cp is always greater than Cv.
The specific heat capacity (c) is another related property that is defined as the amount of heat required to raise the temperature of one unit of mass of a substance by one degree Celsius. The specific heat capacity is related to Cv and Cp through the equation:
c = Cv (for solids) or c = Cp (for gases)
This equation assumes that the substance is in a state of constant volume or constant pressure, respectively.
What is Required Cv and Cp
“Required Cv and Cp” is not a term that has a specific meaning in thermodynamics. However, I can provide some general information about how Cv and Cp might be used in practical applications.
In some cases, engineers or scientists may need to calculate the heat capacity of a substance at a specific volume or pressure. For example, if a system is designed to operate at a specific temperature and pressure, it may be necessary to know the heat capacity of the fluid or gas flowing through the system in order to design an appropriate heat exchanger or other component.
In these situations, the “required” Cv and Cp values would simply refer to the specific heat capacities of the substance at the desired volume and pressure. These values would typically be determined experimentally, using calorimetry or other methods, or they might be calculated using theoretical models or equations of state.
It’s worth noting that the heat capacity of a substance can vary significantly with temperature and pressure, so it’s important to use the appropriate Cv and Cp values for the specific conditions of the system in question.
When is Required Cv and Cp
As I mentioned earlier, “Required Cv and Cp” is not a term that has a specific meaning in thermodynamics. However, engineers or scientists may need to calculate or use Cv and Cp values in various situations where they are required.
One example of when Cv and Cp may be required is in the design and analysis of heat exchangers. Heat exchangers are used to transfer heat between fluids or gases, and the efficiency of a heat exchanger depends in part on the heat capacity of the fluids involved. In this case, the required Cv and Cp values would be the specific heat capacities of the fluids at the operating conditions of the heat exchanger.
Another example is in the study of thermodynamic processes, where it may be necessary to calculate the work and heat transfer associated with a particular process. The work and heat transfer are functions of the change in enthalpy and temperature of the substance, which in turn are related to the Cv and Cp values. In this case, the required Cv and Cp values would be the specific heat capacities of the substance at the initial and final temperatures and pressures of the process.
In summary, Cv and Cp values may be required in a variety of engineering and scientific applications where heat transfer and thermodynamic processes are involved. The specific values required would depend on the specific conditions and requirements of the application.
Where is Required Cv and Cp
The term “Required Cv and Cp” may be used in a variety of engineering and scientific contexts where heat transfer, thermodynamics, or fluid mechanics are involved. Some examples of where Cv and Cp values might be required include:
- Design and analysis of heat exchangers: The performance of a heat exchanger depends on the heat transfer rate between the fluids or gases involved, which in turn is affected by their specific heat capacities at the operating conditions of the heat exchanger. Engineers designing heat exchangers may therefore need to determine the required Cv and Cp values for the fluids they are working with.
- Process simulation and optimization: In process industries such as chemical manufacturing, engineers use simulations and models to optimize production processes and reduce costs. These simulations often involve complex thermodynamic calculations, which require accurate Cv and Cp values for the substances being processed.
- Combustion analysis: Combustion processes involve the transfer of heat and work between a fuel and an oxidizing agent, and the efficiency of the process depends in part on the specific heat capacities of the substances involved. Engineers analyzing combustion processes may need to determine the required Cv and Cp values for the fuel and oxidizer, as well as any combustion products.
- Aerodynamic analysis: The performance of aircraft, rockets, and other vehicles depends on their ability to generate and control airflow. Engineers analyzing the aerodynamics of these systems may need to determine the required Cv and Cp values for the gases involved in order to model their behavior accurately.
In general, any application that involves heat transfer, thermodynamics, or fluid mechanics may require the use of Cv and Cp values in order to accurately model or analyze the system in question. The specific values required will depend on the specific conditions and requirements of the application.
How is Required Cv and Cp
The determination of required Cv and Cp values can involve experimental measurements or theoretical calculations, depending on the nature of the application and the available data.
Experimental methods for measuring Cv and Cp typically involve calorimetry, which involves measuring the heat transfer associated with a change in temperature or pressure. For example, to determine the specific heat capacity at constant volume (Cv) of a solid material, one might use a calorimeter to measure the heat transferred as the material is heated up to a known temperature. The specific heat capacity can then be calculated by dividing the measured heat transfer by the mass of the material and the temperature change.
Similarly, to determine the specific heat capacity at constant pressure (Cp) of a gas or liquid, one might use a bomb calorimeter or flow calorimeter to measure the heat transfer associated with a change in temperature or pressure under controlled conditions. The specific heat capacity can then be calculated as before.
Theoretical methods for calculating Cv and Cp values typically involve using equations of state or thermodynamic models to estimate the properties of the substance under specific conditions. For example, one might use the ideal gas law or the Van der Waals equation of state to calculate the Cv and Cp values of a gas at a given temperature and pressure.
In some cases, empirical correlations or data tables may be available for estimating Cv and Cp values based on known properties of the substance, such as its molecular weight or critical temperature.
Ultimately, the method used to determine the required Cv and Cp values will depend on the specific application and the available data. In many cases, a combination of experimental measurements and theoretical calculations may be used to obtain the most accurate results.
Production of Cv and Cp
Cv and Cp are thermodynamic properties of substances that relate to their ability to store and transfer heat. These properties are intrinsic to the substance and depend on its molecular structure and composition, as well as the conditions under which it is measured or calculated.
The production of Cv and Cp values for a given substance typically involves experimental measurements or theoretical calculations, as I mentioned in my previous answer. Here are some more details about the production of Cv and Cp:
Experimental production of Cv and Cp: To determine the specific heat capacity at constant volume (Cv) or at constant pressure (Cp) of a substance, experimental methods such as calorimetry can be used. Calorimetry is a technique that measures the heat transfer associated with a change in temperature or pressure. The specific heat capacity can then be calculated by dividing the measured heat transfer by the mass of the substance and the temperature or pressure change. Calorimeters can be designed to measure heat transfer under a variety of conditions, including constant volume and constant pressure.
Theoretical production of Cv and Cp: Theoretical methods for calculating Cv and Cp values typically involve using equations of state or thermodynamic models to estimate the properties of the substance under specific conditions. These models may be based on fundamental principles such as the laws of thermodynamics and statistical mechanics, or on empirical correlations derived from experimental data. Some common equations of state used to calculate Cv and Cp values include the ideal gas law, the Van der Waals equation of state, and the Redlich-Kwong equation.
Data tables: Empirical correlations or data tables may be available for estimating Cv and Cp values based on known properties of the substance, such as its molecular weight or critical temperature. These data tables are typically based on experimental measurements or theoretical calculations and can be useful for obtaining estimates of Cv and Cp without performing new experiments or calculations.
In summary, the production of Cv and Cp values involves experimental measurements or theoretical calculations, depending on the nature of the substance and the available data. Empirical correlations or data tables may also be used to estimate Cv and Cp values based on known properties of the substance.
Case Study on Cv and Cp
Here is a case study that illustrates the importance of Cv and Cp in the design of a heat exchanger:
Case study: Design of a heat exchanger for a petrochemical plant
A petrochemical plant produces a variety of chemicals that require heat transfer during their manufacturing processes. To optimize production and reduce costs, the plant is considering the installation of a new heat exchanger to transfer heat between two process streams.
The process streams involved in the heat transfer have different temperatures and specific heat capacities, which affects the heat transfer rate between them. The plant engineers need to determine the required Cv and Cp values for the process streams in order to design an efficient and effective heat exchanger.
To determine the required Cv and Cp values, the engineers perform experimental measurements using a calorimeter. They measure the heat transfer associated with a change in temperature for each process stream at constant volume and constant pressure, respectively. The specific heat capacities can then be calculated by dividing the measured heat transfer by the mass of the substance and the temperature change.
Based on the experimental measurements, the engineers determine that the specific heat capacity at constant volume (Cv) for process stream A is 0.60 kJ/kg-K and the specific heat capacity at constant pressure (Cp) is 0.75 kJ/kg-K. The specific heat capacity at constant volume (Cv) for process stream B is 0.55 kJ/kg-K and the specific heat capacity at constant pressure (Cp) is 0.65 kJ/kg-K.
With these values, the engineers can design a heat exchanger that will transfer heat between the process streams at an optimal rate. By using the Cv and Cp values to calculate the heat transfer coefficient for each process stream, the engineers can optimize the design of the heat exchanger to minimize the temperature difference between the streams and maximize the heat transfer rate.
The engineers also use the Cv and Cp values to calculate the overall heat transfer coefficient for the heat exchanger, which takes into account the thermal resistance of the exchanger itself. This allows them to estimate the overall effectiveness of the heat exchanger and predict its performance under different operating conditions.
In summary, the determination of required Cv and Cp values is essential for the design and optimization of heat exchangers and other thermal systems. These values provide critical information about the heat transfer properties of the substances involved, which is essential for achieving efficient and effective heat transfer.
White paper on Cv and Cp
Here is a white paper that provides an in-depth overview of Cv and Cp and their significance in thermodynamics and engineering applications:
Introduction:
Specific heat capacity (C) is a thermodynamic property that describes the amount of heat energy required to raise the temperature of a substance by one unit. The specific heat capacity of a substance can vary depending on the conditions under which it is measured or calculated. Two commonly used specific heat capacity values are the specific heat capacity at constant volume (Cv) and the specific heat capacity at constant pressure (Cp).
Cv and Cp are fundamental thermodynamic properties that are used to describe the behavior of substances under different conditions. These properties are intrinsic to the substance and depend on its molecular structure and composition. Cv and Cp are important in a wide range of applications, including the design and optimization of thermal systems, the development of thermodynamic models and equations of state, and the study of chemical and physical processes.
Definition and significance of Cv and Cp:
Cv is defined as the specific heat capacity of a substance at constant volume. It represents the amount of heat energy required to raise the temperature of a substance by one unit while keeping its volume constant. Cv is an important property in thermodynamics because it relates to the internal energy (U) of a substance. The internal energy of a substance is the sum of its kinetic and potential energies and is a measure of the total energy stored in the substance.
Cp is defined as the specific heat capacity of a substance at constant pressure. It represents the amount of heat energy required to raise the temperature of a substance by one unit while keeping its pressure constant. Cp is an important property in thermodynamics because it relates to the enthalpy (H) of a substance. Enthalpy is a thermodynamic property that describes the total energy of a substance, including its internal energy and the energy required to do work on the surroundings.
Applications of Cv and Cp:
Cv and Cp are important properties in a wide range of applications, including:
- Heat transfer and thermal systems: The specific heat capacity of a substance determines how much heat energy is required to raise its temperature, which is important in the design and optimization of heat exchangers, boilers, and other thermal systems. The values of Cv and Cp are used to calculate the heat transfer coefficient and the overall heat transfer coefficient for a given system, which are critical in determining its effectiveness and efficiency.
- Thermodynamic models and equations of state: Cv and Cp are used in the development of thermodynamic models and equations of state that describe the behavior of substances under different conditions. These models and equations are used to predict the properties of substances under a wide range of conditions, which is important in the development of new materials and technologies.
- Chemical and physical processes: Cv and Cp are important in the study of chemical and physical processes, such as combustion, phase transitions, and chemical reactions. The values of Cv and Cp can be used to calculate the enthalpy and internal energy changes associated with these processes, which can help to understand and optimize their behavior.
Conclusion:
Cv and Cp are fundamental thermodynamic properties that describe the behavior of substances under different conditions. These properties are intrinsic to the substance and depend on its molecular structure and composition. Cv and Cp are important in a wide range of applications, including the design and optimization of thermal systems, the development of thermodynamic models and equations of state, and the study of chemical and physical processes. Understanding the significance of Cv and Cp is essential in the development of new materials and technologies and the optimization of existing systems.
